Abstract
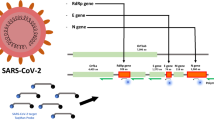
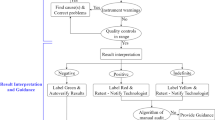
The COVID-19 pandemic has taken the world by surprise and people and organisations worldwide worked in some way or the other to combat the spread; isolate from the infected and get back to normal life, as it was before the pandemic hit. In this regard, the diagnosis of COVID-19 was at the centre of control and prevention and have seen a vehement change in every aspect, especially development of point-of-care testing for better and quick diagnosis. Among different types of techniques developed, the most important was the RT-PCR method of detection which detects nucleic acid of the virus in samples. RT-PCR is a laboratory-based method requiring trained professionals and precise steps for accurate testing. With the advent and spread of the pandemic, number of RT-PCR diagnostic centres rose significantly, and the detection process became less cumbersome, easy to use, ability to handle large volume of samples, more accurate, less time-consuming, and cost-effective. Different industries developed RT-PCR kits, reducing the efforts to prepare laboratory samples. Machines were employed for labour-driven tasks in PCR testing. In addition, new age technologies such as artificial intelligence, IoT, digital systems were combined with RT-PCR for accurate and easy testing. In this review, point-of-care RT-PCR methods, when the COVID-19 started, and the methods now, has been compared on the basis of technological advancements.




Similar content being viewed by others
References
McIntosh K, Hirsch M S, and Bloom A. COVID-19: Epidemiology, virology, and prevention. UpToDate. Available online: https://www.uptodate.com/contents/covid-19-epidemiology-virology-and-prevention (Accessed on 20 Mar 2023).
Eftekhari A, Alipour M, Chodari L, Maleki Dizaj S, Ardalan M, Samiei M, Sharifi S, Zununi Vahed S, Huseynova I, Khalilov R, Ahmadian E, Cucchiarini M. A comprehensive review of detection methods for SARS-CoV-2. Microorganisms. 2021;9(2):232. https://doi.org/10.3390/microorganisms9020232.PMID:33499379;PMCID:PMC7911200.
Available online on: https://covid19.who.int/ (Accessed on 10 Oct 2022).
Sharma S, Shrivastava S, Kausley SB, Rai B, Pandit AB. Coronavirus: a comparative analysis of detection technologies in the wake of emerging variants. Infection. 2022;5(1):1–19.
Teymouri M, Mollazadeh S, Mortazavi H, Ghale-Noie ZN, Keyvani V, Aghababaei F, Mirzaei H. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol Res Pract. 2021;221:153443.
Cupić M, Lazarević I, Knezević A, Stanojević M. Conventional and molecular methods in diagnosis and monitoring of viral infection. Srp Arh Celok Lek. 2007;135(9–10):589–93.
Available online on: https://www.icmr.gov.in/pdf/covid/kits/archive/RT_PCR_Tests_Kits_Evaluation_Summ_17112020.pdf (Accessed on 10 Oct 2022).
Available online on: https://www.finddx.org/covid-19/pipeline/ (Accessed on 10 Oct 2022).
Cho H, Jung YH, Cho HB, Kim HT, Kim KS. Positive control synthesis method for COVID-19 diagnosis by one-step real-time RT-PCR. Clin Chim Acta. 2020;511:149–53. https://doi.org/10.1016/j.cca.2020.10.001.
Available online on: https://www.thehindu.com/sci-tech/health/igib-develops-future-proof-primers-kits-for-rt-pcr-test/article38384374.ece (Accessed on 24 Apr 2023).
Kumar D, Malviya R, Sharma PK. Corona virus: a review of COVID-19. EJMO. 2020;4(1):8–25.
Available online on: https://www.thermofisher.com/blog/clinical-conversations/the-s-gene-advantage-taqpath-covid-19-tests-may-help-with-early-identification-of-omicron-variant/ (Accessed on 24 Apr 2023)
Available online on: https://www.downtoearth.org.in/news/health/what-is-omisure-india-s-first-rt-pcr-kit-that-identifies-omicron-in-under-4-hours-81002 (Accessed on 10 Oct 2022).
Polatoğlu I, Oncu-Oner T, Dalman I, Ozdogan S. COVID-19 in early 2023: Structure, replication mechanism, variants of SARS-CoV-2 diagnostic tests, and vaccine & drug development studies. MedComm. 2023;4(2):e228. https://doi.org/10.1002/mco2.228.
Favaro M, Mattina W, Pistoia ES, Gaziano R, Di Francesco P, Middleton S, D’Angelo S, Altarozzi T, Fontana C. A new qualitative RT-PCR assay detecting SARS-CoV-2. Sci Rep. 2021;11(1):18955. https://doi.org/10.1038/s41598-021-98114-5.
Mardian Y, Kosasih H, Karyana M, Neal A, Lau CY. Review of current COVID-19 diagnostics and opportunities for further development. Front Med. 2021;8:615099.
Zhu N, Wong PK. Advances in viral diagnostic technologies for combating COVID-19 and future pandemics. SLAS Technol. 2020;25(6):513–21. https://doi.org/10.1177/2472630320953798.
Gupta N, Augustine S, Narayan T, O’Riordan A, Das A, Kumar D, Malhotra BD. Point-of-care PCR assays for COVID-19 detection. Biosensors. 2021;11(5):141.
Alanzi T. A review of mobile applications available in the app and google play stores used during the COVID-19 outbreak. J Multidiscip Healthc. 2021;14:45.
Available online on: https://www.thehindu.com/sci-tech/health/igib-develops-future-proof-primers-kits-for-rt-pcr-test/article38384374.ece (Accessed on 20 June 2023).
Blumenfeld NR, Bolene MAE, Jaspan M, et al. Multiplexed reverse-transcriptase quantitative polymerase chain reaction using plasmonic nanoparticles for point-of-care COVID-19 diagnosis. Nat Nanotechnol. 2022;17:984–92. https://doi.org/10.1038/s41565-022-01175-4.
Young RM, Solis CJ, Barriga-Fehrman A, Abogabir C, Thadani AR, Labarca M, Bustamante E, Tapia CV, Sarda AG, Sepulveda F, Pozas N, Cerpa LC, Lavanderos MA, Varela NM, Santibañez A, Sandino AM, Reyes-Lopez F, Dixon G, Quiñones LA. Smartphone screen testing, a novel pre-diagnostic method to identify SARS-CoV-2 infectious individuals. Elife. 2021;10:e70333. https://doi.org/10.7554/eLife.70333.
Available online on: https://www.credodxbiomed.com/en/products/systems/vitapcr (Accessed on 20 June 2023).
Tan M, et al. Recent advances in recombinase polymerase amplification: principle, advantages, disadvantages and applications. Front Cell Infect Microbiol. 2022;12:1744.
Damin F, Galbiati S, Gagliardi S, Cereda C, Dragoni F, Fenizia C, Savasi V, Sola L, Chiari M. CovidArray: a microarray-based assay with high sensitivity for the detection of Sars-Cov-2 in nasopharyngeal swabs. Sensors. 2021;21(7):2490. https://doi.org/10.3390/s21072490.PMID:33916661;PMCID:PMC8038375.
Mei X, Lee HC, Diao KY, Huang M, Lin B, Liu C, Xie Z, Ma Y, Robson PM, Chung M, Bernheim A. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nat Med. 2020;26(8):1224–8. https://doi.org/10.1038/s41591-020-0931-3.
Gomes JC, Masood AI, Silva LHDS, da Cruz Ferreira JRB, Freire Junior AA, Rocha ALDS, Dos Santos WP. Covid-19 diagnosis by combining RT-PCR and pseudo-convolutional machines to characterize virus sequences. Sci Rep. 2021;11(1):1–28.
Horejs C. Artificially intelligent nanopore for rapid SARS-CoV-2 testing. Nat Rev Mater. 2021;6(8):650–650.
Nasajpour M, Pouriyeh S, Parizi RM, Dorodchi M, Valero M, Arabnia HR. Internet of things for current COVID-19 and future pandemics: an exploratory study. J Healthc Inform Res. 2020;4(4):325–64.
Zhu H, Podesva P, Liu X, Zhang H, Teply T, Xu Y, Neuzil P. IoT PCR for pandemic disease detection and its spread monitoring. Sens Actuat B: Chem. 2020;303:127098.
Available online on: https://www.thermofisher.com/in/en/home/life-science/pcr/digital-pcr/quantstudio-absolute-q-system.html (Accessed on 10 Oct 2022).
Vasudevan HN, Xu P, Servellita V, Miller S, Liu L, Gopez A, Abate AR. Digital droplet PCR accurately quantifies SARS-CoV-2 viral load from crude lysate without nucleic acid purification. Sci Rep. 2021;11(1):1–9.
In vitro diagnostics EUAs. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19 -emergency-use-authorizations-medical-devices/vitro-diagnostics-euas (Accessed on 10 Feb 2021).
Chen X, Liu Y, Zhan X, Gao Y, Sun Z, Wen W, Zheng W. Ultrafast PCR detection of COVID-19 by using a microfluidic chip-based system. Bioengineering. 2022;9(10):548. https://doi.org/10.3390/bioengineering9100548.PMID:36290516;PMCID:PMC9598518.
Bukkitgar SD, Shetti NP, Aminabhavi TM. Electrochemical investigations for COVID-19 detection-a comparison with other viral detection methods. Chem Eng J. 2021;420:127575.
Chaibun T, Puenpa J, Ngamdee T, Boonapatcharoen N, Athamanolap P, O’Mullane AP, Lertanantawong B. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nat Commun. 2021;12(1):1–10.
Ji M, Xia Y, Loo JFC, Li L, Ho HP, He J, Gu D. Automated multiplex nucleic acid tests for rapid detection of SARS-CoV-2, influenza A and B infection with direct reverse-transcription quantitative PCR (dirRT-qPCR) assay in a centrifugal microfluidic platform. RSC Adv. 2020;10(56):34088–98.
Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, Orkin CM. Monkeypox virus infection in humans across 16 countries—April–June 2022. New Engl J Med. 2022;387(8):679–91.
Available online on: https://www.who.int/news-room/fact-sheets/detail/monkeypox (Accessed on 10 Oct 2022).
Available online on : https://health.economictimes.indiatimes.com/news/diagnostics/trivitron-healthcare-announces-development-of-real-time-pcr-based-kit-for-monkeypox-virus/91834856 (Accessed on 16 Oct 2022).
Available online on : https://www.einnews.com/pr_news/588282670/digital-pcr-and-real-time-pcr-qpcr-market-future-business-opportunities-2022-2028-abbott-laboratories (Accessed on 16 Oct 2022).
Available online on https://www.iaea.org/bulletin/how-is-the-covid-19-virus-detected-using-real-time-rt-pcr (Accessed on 20 June 2023).
Byrnes SA, Gallagher R, Steadman A, Bennett C, Rivera R, Ortega C, Motley ST, Jain P, Weigl BH, Connelly JT. Multiplexed and extraction-free amplification for simplified SARS-CoV-2 RT-PCR tests. Anal Chem. 2021;93(9):4160–5.
Available online on https://www.biospectrumindia.com/news/97/20598/tata-mds-upgraded-omisure-detects-all-omicron-variants.html (Accessed on 20 June 2023).
Nguyen PQ, Wang M, Ann Maria N, Li AY, Tan HY, Xiong GM, Tan MK, Bhagat AA, Ong CW, Lim CT. Modular micro-PCR system for the onsite rapid diagnosis of COVID-19. Microsyst Nanoeng. 2022;8(1):82. https://doi.org/10.1038/s41378-022-00400-3.
Available online on https://www.medicaldesignbriefs.com/component/content/article/mdb/insiders/mdb/stories/46575 (Accessed on 20 June 2023).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, S., Shrivastava, S., Kausley, S.B. et al. Integrated point-of-care RT-PCR methods during and after COVID-19 pandemic. VirusDis. 34, 356–364 (2023). https://doi.org/10.1007/s13337-023-00834-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-023-00834-x