Abstract
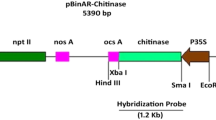
The absence of resistance genes against biotic stresses like Tobacco streak virus (TSV) within compatible peanut germplasm necessitates the deployment of genetic engineering strategy to develop transgenic resistance. Transgenic resistance in peanut (Arachis hypogaea L.) to peanut stem necrosis disease caused by TSV was obtained by transferring coat protein (CP) gene of TSV through Agrobacterium-mediated transformation of de-embryonated cotyledons and immature leaves of peanut cultivars Kadiri 6 (K6) and Kadiri 134 (K134). Integration of the transgene in T1, T2 and T3 generations were confirmed by PCR with gene-specific primers. On the basis of segregation analysis of the PCR amplicons, homozygosity was confirmed in progeny from five transgenic lines. Six transgenic plants from three different single copy transgenic lines homozygous for the transgene were selected for challenge inoculation in T3 generations. The transgenic lines remained symptomless throughout and showed traces or no systemic accumulation of virus indicating the tolerance/resistance to the TSV infection. CP gene expression was observed in transgenic lines by RT-PCR, real-time PCR and ELISA. The findings provide an effective strategy for developing peanut with resistance to peanut stem necrosis disease.







Similar content being viewed by others
References
Bag S, Singh RS, Jain RK. Further analysis of coat protein gene sequences of Tobacco streak virus isolates from diverse locations and hosts in India. Indian Phytopathol. 2008;61:118–23.
Baulcombe DC. Mechanisms of pathogen-derived resistance to viruses in transgenic plants. Plant Cell. 1996;8:1833–44.
Beachy RN. Mechanisms and applications of pathogen-derived resistance in transgenic plants. Curr Opin Biotechnol. 1997;8:215–20.
Bendahmane M, Chen I, Asurmendi S, Bazzini AA, Szecsi J, Beachy RN. Coat protein-mediated resistance to TMV infection of Nicotiana tabacum involves multiple modes of interference by coat protein. Virology. 2007;366:107–16.
Gielen J, Ultzen T, Bontems S, Loots W, Schepen A, Westerbock A, Haan P, van Grinsven M. Coat protein mediated protection of Cucumber mosaic virus infection in cultivated tomato. Euphytica. 1996;88:139–49.
Gonsalves D, Slightom JL. Coat-protein mediated protection: analysis of transgenic plants for resistance in a variety of crops. Semin Virol. 1993;4:397–406.
Hefferon KL, Khalilian H, AbouHaidar MG. Expression of the PVY(O) coat protein (CP) under the control of the PVX CP gene leader sequence: protection under greenhouse and field conditions against PVY(O) and PVY(N) infection in three potato cultivars. Theor Appl Genet. 1997;94(2):287–92.
Hobbs HA, Reddy DVR, Rajeswari R, Reddy AS. Use of direct antigen coating and protein A coating ELISA procedures for detection of three peanut viruses. Plant Dis. 1987;71:747–9.
Höfgen R, Willmitzer L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 1988;16:9877.
Kay R, Chan A, Daly M, McPherson J. Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science. 1987;236:1299–302.
Li ZJ, Jarret RL, Demski JW. Engineered resistance to tomato spotted wilt virus in transgenic peanut expressing the viral nucleocapsid gene. Transgenic Res. 1997;6:297–305.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR & the \( 2^{\Updelta\Updelta {\rm C}_{\rm T}} \) method. Methods. 2001;25:402–8.
Nakajima M, Hayakawa T, Nakamura I, Suzuki M. Protection against cucumber mosaic virus (CMV) strains O and Y and chrysanthemum mild mottle virus in transgenic tobacco plants expressing CMV-O coat protein. J Gen Virol. 1993;74:319–22.
Nishibayashi S, Hayakawa T, Nakajima T, Suzuki M, Kaneko H. CMV protection in transgenic cucumber plants with an introduced CMV-O cp gene. Theor Appl Genet. 1996;93:672–8.
Powell-Abel P, Nelson RS, De B, Hoffmann N, Rogers SG, Fraley RT, Beachy RN. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986;232:738–43.
Prasada Rao RDVJ, Reddy AS, Chander Rao AS, Varaprasad KS, Thirumala Devi K, Nagaraju Muniyappa V, Reddy DVR. Tobacco streak ilarvirus as a causal agent of sunflower necrosis disease in India. J Oilseeds Res. 2000;17:400–1.
Radhakrishnan T, Murthy TGK, Chandran K, Bandyopadhyay A. Micropropagation in peanut (A. hypogaea L.). Biol Plant. 2000;43:447–50.
Reddy AS, Prasad Rao RDVJ, Thirimala Devi K, Reddy SV, Maya MA, Roberts I, Satyanarayana T, Subramaniam K, Reddy DVR. Occurrence of Tobacco streak virus on peanut (Arachis hypogaea) in India. Plant Dis. 2002;86:173–8.
Reimann-Phillipp U. Mechanism of resistance: expression of coat protein. In: Foster GD, Taylor SC, editors. Methods in molecular biology, plant virology protocols: from virus isolation to transgenic resistance, vol. 81. Humana Press: New Jersey; 1998. p. 521–32.
Sambrook J, Russell DW. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2001.
Srivastava A, Raj SK. Coat protein-mediated resistance against an Indian isolate of the Cucumber mosaic virus subgroup IB in Nicotiana benthamiana. J Biosci. 2008;33:249–57.
Taliansky ME, Garcia AF. Role of Cucumovirus capsid protein in long-distance movement within the infected plants. J Virol. 1995;69:916–22.
van Dun CM, Overduin B, van Vloten-Doting L, Bol JL. Transgenic tobacco expressing tobacco streak virus or mutated alfalfa mosaic virus coat protein does not cross protect against alfalfa mosaic virus infection. Virology. 1988;164:383–9.
Acknowledgments
The Department of Biotechnology, Government of India is gratefully acknowledged for financial support to this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehta, R., Radhakrishnan, T., Kumar, A. et al. Coat protein-mediated transgenic resistance of peanut (Arachis hypogaea L.) to peanut stem necrosis disease through Agrobacterium-mediated genetic transformation. Indian J. Virol. 24, 205–213 (2013). https://doi.org/10.1007/s13337-013-0157-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-013-0157-9