Abstract
Background and Objective
A model-informed drug development (MIDD) approach was implemented for paliperidone palmitate (PP) 6-month (PP6M) clinical development, using pharmacokinetics and pharmacokinetic/pharmacodynamic model-based simulations.
Methods
PP6M pharmacokinetics were simulated by extending the PP 3-month (PP3M) pharmacokinetic model to account for increased injection volume, and hence dose. Contribution of the MIDD approach to the design of the pivotal PP6M phase-3 study (PP6M/PP3M noninferiority study, NCT03345342) investigating schizophrenia relapse rates was twofold: (1) PP6M dose selection, and (2) hypothesis generation that lower trough concentrations (Ctrough) associated with PP6M, relative to PP3M, were not associated with lower efficacy, which was to be evaluated in the phase-3 study. Moreover, accompanied by an intense sampling scheme to adequately characterize paliperidone pharmacokinetics and to elucidate the potential relationship between concentration and safety/efficacy, the bridging strategy eliminated the need for additional phase-1/phase-2 clinical studies.
Results
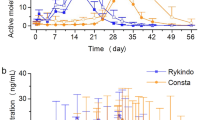
Using a MIDD bridging strategy, PP6M doses were selected that, compared with PP3M, were expected to have a similar range of exposures and a noninferior relapse rate and safety profile. Clinical data from PP6M/PP3M noninferiority study confirmed that PP6M, compared with PP3M, had a similar range of exposures (T’jollyn et al. in Eur J Drug Metab Pharmacokinet 2024), as well as a noninferior relapse rate and safety profile (this manuscript).
Conclusions
Consistency of the MIDD approach with observed clinical outcomes confirmed the hypothesis that lower Ctrough did not lead to increased relapse rates at the doses administered. Although higher paliperidone peak concentrations are achieved with corresponding doses of PP6M relative to PP3M in the phase-3 clinical study, types and incidences of treatment-related adverse events were comparable between PP6M and PP3M groups and no new safety concerns emerged for PP6M (Najarian et al. in Int J Neuropsychopharmacol 25(3):238–251, 2022).






Similar content being viewed by others
References
Najarian D, Sanga P, Wang S, Lim P, Singh A, Robertson MJ, et al. A randomized, double-blind, multicenter, noninferiority study comparing paliperidone palmitate 6-month versus the 3-month long-acting injectable in patients with schizophrenia. Int J Neuropsychopharmacol. 2022;25(3):238–51.
Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16(4):505–24.
Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry. 2009;52:S63–7.
Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–26.
Kishimoto T, Hagi K, Nitta M, Leucht S, Olfson M, Kane JM, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603–19.
Novick D, Haro JM, Suarez D, Perez V, Dittmann RW, Haddad PM. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176(2):109–13.
Berwaerts J, Liu Y, Gopal S, Nuamah I, Xu H, Savitz A, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiat. 2015;72(8):830–9.
Savitz AJ, Xu H, Gopal S, Nuamah I, Ravenstijn P, Janik A, et al. Efficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter, double-blind, noninferiority study. Int J Neuropsychopharmacol. 2016;19(7):pyw018.
Hough D, Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116(2–3):107–17.
Attard A, Olofinjana O, Cornelius V, Curtis V, Taylor D. Paliperidone palmitate long-acting injection–prospective year-long follow-up of use in clinical practice. Acta Psychiatr Scand. 2014;130(1):46–51.
Bioque M, Bernardo M. The current data on the 3-month paliperidone palmitate formulation for the treatment of schizophrenia. Expert Opin Pharmacother. 2018;19(14):1623–9.
Mathews M, Gopal S, Nuamah I, Hargarter L, Savitz AJ, Kim E, et al. Clinical relevance of paliperidone palmitate 3-monthly in treating schizophrenia. Neuropsychiatr Dis Treat. 2019;15:1365–79.
Hafyera (paliperidone) [prescribing information]. Janssen Pharmaceutical Companies. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/207946s010lbl.df . Accessed 2 Feb 2024.
Byannli (paliperidone) [EPAR]. Janssen Cilag International. https://www.ema.europa.eu/en/documents/product-information/byannli-previously-paliperidone-janssen-cilag-international-epar-product-information_en.pdf. Accessed 2 Feb 2024.
Madabushi R, Seo P, Zhao L, Tegenge M, Zhu H. Review: role of model-informed drug development approaches in the lifecycle of drug development and regulatory decision-making. Pharm Res. 2022;39(8):1669–80.
Magnusson MO, Samtani MN, Plan EL, Jonsson EN, Rossenu S, Vermeulen A, et al. Population pharmacokinetics of a novel once-every 3 months intramuscular formulation of paliperidone palmitate in patients with schizophrenia. Clin Pharmacokinet. 2017;56(4):421–33.
Russu A, Savitz A, Mathews M, Gopal S, Feng Y, Samtani MN. Pharmacokinetic-pharmacodynamic characterization of relapse risk for paliperidone palmitate 1-month and 3-month formulations. J Clin Psychopharmacol. 2019;39(6):567–74.
Samtani MN, Vermeulen A, Stuyckens K. Population pharmacokinetics of intramuscular paliperidone palmitate in patients with schizophrenia: a novel once-monthly, long-acting formulation of an atypical antipsychotic. Clin Pharmacokinet. 2009;48(9):585–600.
T’jollyn H, Venkatasubramaniam R, Neyens M, Gopal S, Russu A, Nandy P, et al. Model-informed clinical development of once-every 6-month injection of paliperidone palmitate in patients with schizophrenia: dosing strategies guided by population pharmacokinetic modeling and simulation (part II). Eur J Drug Metab Pharmacokinet. 2024 (under review).
Savitz AJ, Xu H, Gopal S, Nuamah I, Ravenstijn P, Hough D, et al. Efficacy and safety of paliperidone palmitate three-monthly formulation in East Asian patients with schizophrenia: subgroup analysis of a global, randomized, double-blind, Phase III, noninferiority study. Neuropsychiatr Dis Treat. 2017;13:2193–207.
Savitz AJ, Xu H, Gopal S, Nuamah I, Ravenstijn P, Hough D, et al. Efficacy and safety of paliperidone palmitate 3-month versus 1-month formulation in patients with schizophrenia: comparison between European and non-European population. Neuropsychiatr Dis Treat. 2019;15:587–602.
Savitz AJ, Xu H, Gopal S, Nuamah I, Mathews M, Soares B. Efficacy and safety of paliperidone palmitate 3-month formulation in Latin American patients with schizophrenia: a subgroup analysis of data from two large phase 3 randomized, double-blind studies. Braz J Psychiatry. 2019;41(6):499–510.
Acknowledgements
Writing assistance was provided Uma Kundu, MPharm, CMPPTM (SIRO Clinpharm Pvt. Ltd.) and funded by Janssen Global Services, LLC and additional editorial support for this manuscript was provided by Ellen Baum, PhD (Janssen Global Services, LLC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Contributions
SG, AR, PN, and JJPR conceptualized and designed the study; SG RV performed data collection; HT, AR, RV, OA, PN, and JJPR performed data analysis and interpretation; HT and AR performed manuscript writing. All authors reviewed and provided final approval for the manuscript and are accountable for all aspects of the work.
Funding
Janssen Research & Development, LLC, USA.
Conflict of Interest
Srihari Gopal was employed by Janssen Research & Development, LLC, USA when the study was conducted and is currently employed by Regeneron Pharmaceuticals, USA. Partha Nandy was employed by Janssen Research & Development, LLC, USA when the study was conducted and is currently employed by CSL Behring, USA. All other authors are employees of Janssen Research & Development, LLC (a Johnson & Johnson company) and hold stock in Johnson & Johnson.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and in adherence with the Good Clinical Practice guidelines and was approved by the institutional review board (as listed in the original research paper by Najarian et al.: A randomized, double-blind, multicenter, noninferiority study comparing paliperidone palmitate 6-month versus the 3-month long-acting injectable in patients with schizophrenia).
Informed consent
Informed consent was obtained from all patients before enrollment in the original study.
Consent for publication
Not applicable.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access [YODA] Project site at http://yoda.yale.edu.
Code Availability
The model equations are included in the Supplementary Material.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
T’jollyn, H., Russu, A., Venkatasubramanian, R. et al. Model-Informed Clinical Development of Once-Every-6-Month Injection of Paliperidone Palmitate in Patients with Schizophrenia: A Pharmacometric Bridging Approach (Part I). Eur J Drug Metab Pharmacokinet (2024). https://doi.org/10.1007/s13318-024-00900-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s13318-024-00900-9