Abstract
Background and Objectives
Tetramethylpyrazine nitrone (TBN) is a novel tetramethylpyrazine derivative armed with a strong free radical scavenging nitrone moiety. This study aims to evaluate the pharmacokinetics, safety profile, and tolerability of TBN tablets after a single ascending dose (SAD) and multiple ascending doses (MAD) in healthy Chinese volunteers.
Methods
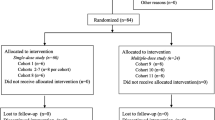
This phase I, single-center, open-label study was conducted in China. The SAD portion consisted of four cohorts with dose levels of 400–1800 mg. The MAD portion included three cohorts in which subjects received doses of 600–1800 mg twice daily for 7 days (13 consecutive doses). The third portion was a randomized, two-period, crossover design to assess the influence of food with a single dose of TBN tablets (1200 mg). The safety profile was evaluated by monitoring adverse events (AEs), vital signs, electrocardiograms, physical examinations, and laboratory test results.
Results
Fifty-two healthy subjects aged 18 to 45 years with a body mass index between 19.0 and 26.0 kg/m2 were enrolled. After a single dose of TBN, the median time to maximum plasma concentration (Tmax) was 2.48–3.24 h and the mean half-life (t1/2) was 1.28 to 2.10 h across all doses. In the MAD study, the median Tmax was 2.48 to 3.48 h. In the 400–1800 mg dose range, there was a tendency for less than proportional increases in the maximum plasma concentration (Cmax), the area under the concentration-time curve from 0 to time of last measurable concentration (AUC0–t), and the area under the concentration-time curve from 0 to infinity (AUC0–inf) in both single- and multiple-dose periods. A significantly higher TBN exposure was observed in females than males in both a single and multiple doses of the 600 mg and 1200 mg groups, with a geometric mean female-to-male ratio of 138.69–203.18%. Food decreased the Cmax and AUC0–t of TBN to 45.19% and 59.73%, respectively. Each dose group reached a steady state after 4 days. No drug accumulation was observed. Two subjects had drug-related AEs. A decreased neutrophil count and drug eruption in the SAD portion (1200 mg group) and an increased alanine aminotransferase level in the food effect group were found. All AEs were mild and tolerable (CTCAE grade 1) and resolved without any medical intervention.
Conclusion
TBN tablets had a good safety profile and were well tolerated in healthy Chinese volunteers. Steady-state concentrations were reached after 4 consecutive days of oral administration. The results of this phase I study will provide guidance for the design of future TBN clinical studies.
Chinese Clinical Trial Registry
ChiCTR1900022092.


Similar content being viewed by others
References
Johansen KL, Chertow GM, Foley RN, Gilbertson DT, Herzog CA, Ishani A, et al. US renal data system 2020 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2021;77(4 Suppl 1):A7–8. https://doi.org/10.1053/j.ajkd.2021.01.002.
Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. https://doi.org/10.1056/NEJMoa052187.
Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16(4):206–22. https://doi.org/10.1038/s41581-019-0234-4.
Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5(1):49–56.
Paul Drawz MR. Chronic kidney disease. Ann Intern Med. 2015;162(11):ITC1–16. https://doi.org/10.7326/aitc201506020%m26030647
Yakush Williams JK. Management strategies for patients with diabetic kidney disease and chronic kidney disease in diabetes. Nurs Clin N Am. 2017;52(4):575–87. https://doi.org/10.1016/j.cnur.2017.07.007.
Ricciardi CA, Gnudi L. Kidney disease in diabetes: from mechanisms to clinical presentation and treatment strategies. Metabolism. 2021;124: 154890. https://doi.org/10.1016/j.metabol.2021.154890.
Lytvyn Y, Bjornstad P, van Raalte DH, Heerspink HL, Cherney DZI. The new biology of diabetic kidney disease-mechanisms and therapeutic implications. Endocr Rev. 2020;41(2):202–31. https://doi.org/10.1210/endrev/bnz010.
Jha JC, Banal C, Chow BS, Cooper ME, Jandeleit-Dahm K. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25(12):657–84. https://doi.org/10.1089/ars.2016.6664.
Pan D, Zhang D, Wu J, Chen C, Xu Z, Yang H, et al. A novel proteoglycan from Ganoderma lucidum fruiting bodies protects kidney function and ameliorates diabetic nephropathy via its antioxidant activity in C57BL/6 db/db mice. Food Chem Toxicol. 2014;63:111–8. https://doi.org/10.1016/j.fct.2013.10.046.
Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. https://doi.org/10.1016/j.redox.2016.12.022.
Shukla R, Banerjee S, Tripathi YB. Antioxidant and Antiapoptotic effect of aqueous extract of Pueraria tuberosa (Roxb. Ex Willd.) DC. On streptozotocin-induced diabetic nephropathy in rats. BMC Complement Altern Med. 2018;18(1):156. https://doi.org/10.1186/s12906-018-2221-x.
Sattarinezhad A, Roozbeh J, Shirazi Yeganeh B, Omrani GR, Shams M. Resveratrol reduces albuminuria in diabetic nephropathy: a randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019;45(1):53–9. https://doi.org/10.1016/j.diabet.2018.05.010.
Golbidi S, Ebadi SA, Laher I. Antioxidants in the treatment of diabetes. Curr Diabetes Rev. 2011;7(2):106–25. https://doi.org/10.2174/157339911794940729.
Gong X, Ivanov VN, Davidson MM, Hei TK. Tetramethylpyrazine (TMP) protects against sodium arsenite-induced nephrotoxicity by suppressing ROS production, mitochondrial dysfunction, pro-inflammatory signaling pathways and programed cell death. Arch Toxicol. 2015;89(7):1057–70. https://doi.org/10.1007/s00204-014-1302-y.
Marshall JW, Duffin KJ, Green AR, Ridley RM. NXY-059, a free radical–trapping agent, substantially lessens the functional disability resulting from cerebral ischemia in a primate species. Stroke. 2001;32(1):190–8. https://doi.org/10.1161/01.str.32.1.190.
Zhang G, Zhang T, Li N, Wu L, Gu J, Li C, et al. Tetramethylpyrazine nitrone activates the BDNF/Akt/CREB pathway to promote post-ischaemic neuroregeneration and recovery of neurological functions in rats. Br J Pharmacol. 2018;175(3):517–31. https://doi.org/10.1111/bph.14102.
Jing M, Cen Y, Gao F, Wang T, Jiang J, Jian Q, et al. Nephroprotective effects of tetramethylpyrazine nitrone TBN in diabetic kidney disease. Front Pharmacol. 2021;12: 680336. https://doi.org/10.3389/fphar.2021.680336.
Wu L, Su Z, Zha L, Zhu Z, Liu W, Sun Y, et al. Tetramethylpyrazine nitrone reduces oxidative stress to alleviate cerebral vasospasm in experimental subarachnoid hemorrhage models. Neuromol Med. 2019;21(3):262–74. https://doi.org/10.1007/s12017-019-08543-9.
Li S. A blinded, multicenter, randomized phase II trial of tetramethylpyrazine nitrone in patients with acute ischemic stroke. In: Paper presented at the 8th congress of the European Academy of Neurology. 2022.
Zhao C, Lv Y, Cui H, Zhu Y, Wei M, Xia Y, et al. Phase I safety, tolerability, and pharmacokinetic studies of tetramethylpyrazine nitrone in healthy Chinese volunteers. Drug Dev Res. 2021;82(1):97–107. https://doi.org/10.1002/ddr.21733.
Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17(10):1278–83. https://doi.org/10.1023/a:1026451721686.
Sun Y, Yu P, Zhang G, Wang L, Zhong H, Zhai Z, et al. Therapeutic effects of tetramethylpyrazine nitrone in rat ischemic stroke models. J Neurosci Res. 2012;90(8):1662–9. https://doi.org/10.1002/jnr.23034.
Zhang G, Zhang T, Wu L, Zhou X, Gu J, Li C, et al. Neuroprotective effect and mechanism of action of tetramethylpyrazine nitrone for ischemic stroke therapy. Neuromol Med. 2018;20(1):97–111. https://doi.org/10.1007/s12017-018-8478-x.
Yonkers KA, Kando JC, Cole JO, Blumenthal S. Gender differences in pharmacokinetics and pharmacodynamics of psychotropic medication. Am J Psychiatry. 1992;149(5):587–95. https://doi.org/10.1176/ajp.149.5.587.
Soldin OP, Chung SH, Mattison DR. Sex differences in drug disposition. J Biomed Biotechnol. 2011;2011: 187103. https://doi.org/10.1155/2011/187103.
Acknowledgements
Many thanks to Ms. Linda Wang of Guangzhou Magpie Pharmaceuticals Co., Ltd., for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
Study concept and design: GZ, LW, and XH. Data acquisition: SZ, and SH. Data analysis and interpretation: SZ, SH, HP, and MT. GZ was primarily responsible for drafting the manuscript and all authors reviewed, edited, and approved the manuscript before submission.
Funding
This work was partially supported by the Scientific Projects of Guangdong Province (2020A050515008); the Technology Innovation Project of Foshan (2017IT100153); and Guangzhou Magpie Pharmaceuticals Co., Ltd.
Conflict of interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Ethics approval
The clinical study met all local legal and regulatory requirements. The studies were conducted under contract with Guangzhou Magpie Pharmaceutical Co., Ltd., by the following research organizations, with approval from their ethical review committees: Haikou People's Hospital (approval number: 2019-(ethical review)-002, approval date: April 18, 2019). All procedures in these studies were performed in accordance with the 1964 Helsinki Declaration (and its amendments).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Consent to participate
Informed consent was obtained from all individual participants prior to the initiation of any study-related procedure.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, G., Wang, L., Zhong, S. et al. Pharmacokinetics, Safety Profile, and Tolerability of Tetramethylpyrazine Nitrone Tablets After Single and Multiple Ascending Doses in Healthy Chinese Volunteers. Eur J Drug Metab Pharmacokinet 49, 207–217 (2024). https://doi.org/10.1007/s13318-024-00877-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-024-00877-5