Abstract
Background and Objective
Acetaminophen (paracetamol) is a ubiquitously administered drug in critically ill patients. Considering the dearth of literature, we evaluated the population pharmacokinetics of intravenous acetaminophen and its principal metabolites (sulfate and glucuronide) in this population.
Methods
Critically ill adults receiving intravenous acetaminophen were included in the study. One to three blood samples were withdrawn per patient for the estimation of acetaminophen, and its metabolites (acetaminophen glucuronide and acetaminophen sulfate). High-performance liquid chromatography was used for measuring serum concentrations. We used nonlinear mixed-effect modeling for estimating the primary pharmacokinetic parameters of acetaminophen and its metabolites. The effect of covariates was evaluated followed by dose optimization using Monte Carlo simulation. Patient factors such as demographic information, liver and renal function tests were used as covariates in population pharmacokinetic analysis. The therapeutic range for serum acetaminophen concentration was considered to be 66–132 μM, while 990 μM was considered as the threshold for toxic concentration.
Results
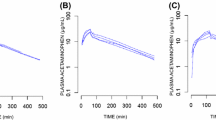
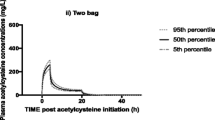
Eighty-seven participants were recruited. A joint two-compartment acetaminophen pharmacokinetic model linked to glucuronide and sulfate metabolite compartments was used. The central and peripheral volume distributions were 7.87 and 8.87 L/70 kg, respectively. Estimated clearance (CL) was 0.58 L/h/70 kg, while intercompartmental clearance was 44.2 L/h/70 kg. The glucuronide and sulfate metabolite CL were 22 and 94.7 L/h/70 kg, respectively. Monte Carlo simulation showed that twice-daily administration of acetaminophen would result in a relatively higher proportion of patient population achieving and retaining serum concentrations in the therapeutic range, with reduced risk of concentrations remaining in the toxic range.
Conclusion
A joint pharmacokinetic model for intravenous acetaminophen and its principal metabolites in a critically ill patient population has been developed. Acetaminophen CL in this patient population is reduced. We propose a reduction in the frequency of administration to reduce the risk of supra-therapeutic concentrations in this population.




Similar content being viewed by others
References
Sridharan K, Hasan H, Al Jufairi M, Al Daylami A, Abdul Azeez Pasha S, Al Ansari E. Drug utilisation in adult, paediatric and neonatal intensive care units, with an emphasis on systemic antimicrobials. Anaesthesiol Intensive Ther. 2021; 53(1): 18-24.
Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J. Introduction to drug pharmacokinetics in the critically iII patient. Chest. 2012;141:1327–36.
Tansley G, Hall R. Pharmacokinetic considerations for drugs administered in the critically ill. Br J Hosp Med. 2015;76:89–94.
Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3–11.
Sridharan K, Pasha SAA, Qader AM, Hasan HMSN, El Seirafi MM. Drug Utilization in critically Ill adults with augmented renal clearance compared to normal renal clearance: implications for use of antimicrobials with predominant renal excretion. Curr Rev Clin Exp Pharmacol. 2021;16(2):174–81.
Freo U, Ruocco C, Valerio A, Scagnol I, Nisoli E. Paracetamol: a review of guideline recommendations. J Clin Med. 2021;10:3420.
White PF. Cost-effective multimodal analgesia in the perioperative period: Use of intravenous vs. oral acetaminophen. J Clin Anesth. 2020; 61: 109625.
Morse JD, Stanescu I, Atkinson HC, Anderson BJ. Population pharmacokinetic modelling of acetaminophen and ibuprofen: the influence of body composition, formulation and feeding in healthy adult volunteers. Eur J Drug Metab Pharmacokinet. 2022. https://doi.org/10.1007/s13318-022-00766-9.
Samson AD, Hunfeld NG, Touw DJ, Melief PH. Efficacy and pharmacokinetics of intravenous paracetamol in the critically ill patient. Crit Care. 2009;13(Suppl 1):P407.
Parker SL, Saxena M, Gowardman J, Lipman J, Myburgh J, Roberts JA. Population pharmacokinetics of intravenous paracetamol in critically ill patients with traumatic brain injury. J Crit Care. 2018;47:15–20.
Geronimo-Pardo M, Fuster-Lluch O, Peyro-Garcia R, Lizan-Garcia M. Paracetamol pharmacokinetics in critically ill trauma patients. Eur J Anaesthesiol. 2010;27:153.
Athersuch TJ, Antoine DJ, Boobis AR, Coen M, Daly AK, Possamai L, Nicholson JK, Wilson ID. Paracetamol metabolism, hepatotoxicity, biomarkers and therapeutic interventions: a perspective. Toxicol Res (Camb). 2018;7(3):347–57.
Horvatits T, Drolz A, Trauner M, Fuhrmann V. Liver injury and failure in critical illness. Hepatology. 2019;70(6):2204–15.
Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003–9.
Chen IH, Nicolau DP. Augmented renal clearance and how to augment antibiotic dosing. Antibiotics (Basel). 2020;9(7):393.
Oscier CD, Milner QJ. Peri-operative use of paracetamol. Anaesthesia. 2009;64(1):65–72.
Sridharan K, Qader AM, Hammad M, Jassim A, Diab DE, Abraham B, Hasan HMSN, Pasha SAA, Shah S. Evaluation of the association between single nucleotide polymorphisms of metabolizing enzymes with the serum concentration of paracetamol and its metabolites. Metabolites. 2022;12(12):1235.
Diab DE, Sridharan K. Development of urinary assay methods for the estimation of paracetamol glucuronide and paracetamol sulfate in preterm neonates with patent ductus arteriosus. Curr Chromat. 2022;9:1–5.
Monolix version 2021R2. Antony, France: Lixoft SAS, 2022. http://lixoft.com/products/monolix/. Accessed 9 October 2022.
Package ‘Rsmlx’. https://cran.r-project.org/web/packages/Rsmlx/Rsmlx.pdf. Accessed 9 Oct 2022.
Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin Toxicol. 2002;40(1):3–20.
Rawlins MD, Henderson DB, Hijab AR. Pharmacokinetics of paracetamol (acetaminophen) after intravenous and oral administration. Eur J Clin Pharmacol. 1977;11(4):283–6.
Acetaminophen injection. Product monograph. Baxter corporation. https://www.baxter.ca/sites/g/files/ebysai1431/files/2021-08/EN_ACETAMINOPHEN%20INJECTION.pdf. Accessed 13 Oct 2022.
Allegaert K, Olkkola KT, Owens KH, Van de Velde M, de Maat MM, Anderson BJ; PACIA study group. Covariates of intravenous paracetamol pharmacokinetics in adults. BMC Anesthesiol 2014; 14: 77.
de Maat MM, Tijssen TA, Brüggemann RJ, Ponssen HH. Paracetamol for intravenous use in medium–and intensive care patients: pharmacokinetics and tolerance. Eur J Clin Pharmacol. 2010;66(7):713–9.
Kluge M, Tacke F. Liver impairment in critical illness and sepsis: the dawn of new biomarkers? Ann Transl Med. 2019;7(Suppl 8):S258.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received to conduct this study.
Data Availability Statement
The data is available with the corresponding author and shall be shared upon a reasonable request.
Conflict of Interest
Kannan Sridharan, Mwila Mulubwa, and Ali Mohamed Qader do not have any conflict of interest.
Ethics Approval
The study was approved by the Institutional Ethics Committee of Arabian Gulf University (E032-PI-9/21 on 17th October 2021), and Salmaniya Medical Complex (126081121 on 8th November 2021), Kingdom of Bahrain.
Consent to participate
Consent to participate in this research study was obtained from the patients or their legally acceptable representatives.
Consent for publication
Not applicable.
Code availability
Not applicable.
Authors' contributions
KS: Conceived the study idea, wrote proposal, and obtained ethics approval; KS, AMQ: Data collection; MW: Model development; KS, MW: Wrote the first draft of the manuscript; KS, MW, AMQ: revised the drafts and final approval of the final version.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sridharan, K., Mulubwa, M. & Qader, A.M. Population Pharmacokinetic Modeling and Dose Optimization of Acetaminophen and its Metabolites Following Intravenous Infusion in Critically ill Adults. Eur J Drug Metab Pharmacokinet 48, 531–540 (2023). https://doi.org/10.1007/s13318-023-00841-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-023-00841-9