Abstract
Background and Objective
Lu-177 DOTATATE (Lutathera®) is a radiolabeled analog of somatostatin administered intravenously in patients with somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors. Biodistribution of Lu-177 DOTATATE in tumor and healthy tissues can be monitored by serial post-injection scintigraphy imaging. Patient exposure to the drug is variable with the recommended fixed dosage, and hence there is a variable response to treatment. The aim of this work was to study the pharmacokinetics of Lu-177 DOTATATE by a population modeling approach, based on single-photon emission computed tomography (SPECT)/computed tomography (CT) images used as surrogate of plasma concentrations to study the interindividual variability and finally optimize an individual dosage.
Methods
From a retrospective study, SPECT/CT images were acquired at 4 h, 24 h, 72 h, and 192 h postadministration. From these images, volumic activities were calculated in blood and bone marrow. An individual non-compartmental pharmacokinetic analysis was performed, and the mean pharmacokinetic parameters of each tissue were compared together and with reference data. Blood volumic activities were then used to perform a population pharmacokinetic analysis (NONMEM).
Results
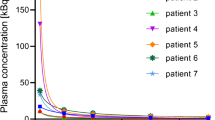
The pharmacokinetic parameters (non-compartmental analysis) obtained from blood (clearance [CL] = 2.65 L/h, volume of distribution at steady state [Vss] = 309 L, elimination half-life [t1/2] = 86.3 h) and bone marrow (CL =1.68 L/h, Vss = 233 L, t1/2 = 98.8 h) were statistically different from each other and from reference values (CL = 4.50 L/h, Vss = 460 L, t1/2 = 71.0 h) published in the literature. SPECT/CT blood images were used as a surrogate of plasma concentrations to develop a population pharmacokinetic model. Weight was identified as covariate on volume of the central compartment, reducing the interindividual variability of all population pharmacokinetic parameters.
Conclusion
This study is a proof of concept that obtaining pharmacokinetic parameters with image-based blood concentration is possible. Obtaining observed concentrations from SPECT/CT images, without the need for blood sampling, is a real advantage for the patient and the drug monitoring. Pharmacokinetic modeling could be combined with a deep learning model for automatic contouring and allow precise patient-specific dose adjustment in a non-invasive manner.




Similar content being viewed by others
References
Hennrich U, Kopka K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals. Multidisciplinary Digital Publishing Institute; 2019;12:114.
Pouget J-P, Santoro L, Piron B, Paillas S, Ladjohounlou R, Pichard A, et al. From the target cell theory to a more integrated view of radiobiology in targeted radionuclide therapy: The Montpellier group’s experience. Nucl Med Biol. 2021;104–105.
Gupta SK, Singla S, Thakral P, Bal CS. Dosimetric analyses of kidneys, liver, spleen, pituitary gland, and neuroendocrine tumors of patients treated with 177Lu-DOTATATE. Clin Nucl Med. 2013;38:188–94.
Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38(12):2125–35.
Sabet A, Ezziddin K, Pape U-F, Ahmadzadehfar H, Mayer K, Pöppel T, et al. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2013;54:1857–61.
Del Prete M, Buteau F-A, Arsenault F, Saighi N, Bouchard L-O, Beaulieu A, et al. Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: initial results from the P-PRRT trial. Eur J Nucl Med Mol Imaging. 2019;46:728–42.
Sundlöv A, Gleisner KS, Tennvall J, Ljungberg M, Warfvinge CF, Holgersson K, et al. Phase II trial demonstrates the efficacy and safety of individualized, dosimetry-based 177Lu-DOTATATE treatment of NET patients. Eur J Nucl Med Mol Imaging. 2022;49:3830–40.
Siebinga H, de Wit-van der Veen BJ, Stokkel MDM, Huitema ADR, Hendrikx JJMA. Current use and future potential of (physiologically based) pharmacokinetic modelling of radiopharmaceuticals: a review. Theranostics. Ivyspring International Publisher; 2022;12:7804–20.
Forrer F, Krenning EP, Kooij PP, Bernard BF, Konijnenberg M, Bakker WH, et al. Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA0, Tyr3]octreotate. Eur J Nucl Med Mol Imaging. 2009;36:1138–46.
Santoro L, Pitalot L, Trauchessec D, Mora-Ramirez E, Kotzki PO, Bardiès M, et al. Clinical implementation of PLANET® Dose for dosimetric assessment after [177Lu]Lu-DOTA-TATE: comparison with Dosimetry Toolkit® and OLINDA/EXM® V1.0. EJNMMI Res. 2021;11:1.
Shen S, Meredith RF, Duan J, Macey DJ, Khazaeli MB, Robert F, et al. Improved prediction of myelotoxicity using a patient-specific imaging dose estimate for non-marrow-targeting (90)Y-antibody therapy. J Nucl Med. 2002;43:1245–53.
PKanalix version 2021R2. Antony, France: Lixoft SAS, 2021. Available from: https://lixoft.com/products/pkanalix/. Access date: 04/04/2022.
FDA, Highlights of prescribing information - LUTATHERA - 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208700s000lbl.pdf. Access date: 04/02/2022.
Nolain P, Combet R, Marchionni D, Speth H, Martinez JM, Fabre D. PopkinR: a suite of Shiny applications focused on the pharmacometrics workflow. Poster presented to Population Approach Group Europe (PAGE) meeting. Switzerland, 2018. https://www.page-meeting.org/?abstract=8684. Access date: 07/04/2023.
Lindbom L, Pihlgren P, Jonsson N. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN): a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75:85–94.
Hagmarker L, Svensson J, Rydén T, van Essen M, Sundlöv A, Gleisner KS, et al. Bone marrow absorbed doses and correlations with hematologic response during 177Lu-DOTATATE treatments are influenced by image-based dosimetry method and presence of skeletal metastases. J Nucl Med. 2019;60:1406–13.
Puszkiel A, Bauriaud-Mallet M, Bourgeois R, Dierickx L, Courbon F, Chatelut E. Evaluation of the interaction of amino acid infusion on 177Lu-dotatate pharmacokinetics in patients with gastroenteropancreatic neuroendocrine tumors. Clin Pharmacokinet. 2019;58:213–22.
Jiménez-Franco LD, Glatting G, Prasad V, Weber WA, Beer AJ, Kletting P. Effect of tumor perfusion and receptor density on tumor control probability in 177Lu-DOTATATE therapy: an in silico analysis for standard and optimized treatment. J Nucl Med. 2021;62:92–8.
Chan Kwong AH-XP, O'Jeanson A and Khier S. Model-informed therapeutic drug monitoring of meropenem in critically ill patients: improvement of the predictive ability of literature models with the PRIOR approach. Eur J Drug Metab Pharmacokinet. 2021 ;46(3):415-426.
Chan Kwong AH-XP, Calvier EAM, Fabre D, Gattacceca F, Khier S. Prior information for population pharmacokinetic and pharmacokinetic/pharmacodynamic analysis: overview and guidance with a focus on the NONMEM PRIOR subroutine. J Pharmacokinet Pharmacodyn. 2020;47:431–46.
Lutathera European public assessment report. https://www.ema.europa.eu/en/documents/assessment-report/lutathera-epar-public-assessment-report_en.pdf. Accessed on 02 July 2022.
Lubberink M, Wilking H, Öst A, Ilan E, Sandström M, Andersson C, et al. In vivo instability of 177 Lu-DOTATATE during peptide receptor radionuclide therapy. J Nucl Med. 2020;61(9):1337–40.
Ljungberg M, Celler A, Konijnenberg MW, Eckerman KF, Dewaraja YK, Sjögreen-Gleisner K. MIRD Pamphlet No. 26: Joint EANM/MIRD Guidelines for Quantitative 177Lu SPECT applied for dosimetry of radiopharmaceutical therapy. J Nucl Med. 2016;57(1):151-62.
Acknowledgements
Dr Anna Chan Kwong, Dr David Fabre, and all the Pharmacokinetics modeling and Pharmacometrics team (Sanofi Montpellier) for their advice on modeling and for the use of NONMEM license. Ms Souad Mekki and Ms Ikrame Berkane, Master degree students, for their help in data collection, image reconstruction, and contouring.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no financial nor non-financial interests that are directly or indirectly related to the work submitted for publication.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Author contributions
AB extracted and collected the data, analyzed the data, analyzed literature, and wrote the initial draft of the manuscript. LS contributed to the literature search, data extraction, and writing of the manuscript. MV supervised the statistical analysis and reviewing the manuscript. POK contributed to reviewing the manuscript. ED contributed to data extraction, provided clinical input, and reviewed the manuscript. SK conceptualized the study, contributed to literature research, supervised the pharmacokinetic analysis, and wrote the manuscript.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Not applicable.
Code availability
May be available upon reasonable request from the corresponding author.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barakat, A., Santoro, L., Vivien, M. et al. Clinical Pharmacokinetics of Radiopharmaceuticals from SPECT/CT Image Acquisition by Contouring in Patients with Gastroenteropancreatic Neuroendocrine Tumors: Lu-177 DOTATATE (Lutathera®) Case. Eur J Drug Metab Pharmacokinet 48, 329–339 (2023). https://doi.org/10.1007/s13318-023-00829-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-023-00829-5