Abstract
Background and Objective
Tacrolimus has become the first-line immunosuppressant for preventing rejection after heart transplantation. The present study aimed to investigate genetic variants and clinical factors affecting the variability of tacrolimus in Chinese Han heart transplant patients using a population pharmacokinetic approach.
Methods
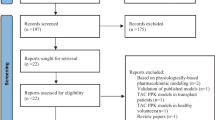
The retrospective study included 53 hospitalized patients with 547 tacrolimus concentrations for analysis. Nonlinear mixed-effects modeling was used to develop the population pharmacokinetics model for tacrolimus in patients with heart transplants, followed by Monte Carlo simulations to design initial dosing regimens.
Results
In our study, the mutation rate of CYP3A4*18B (C>T) was 27.36%. An oral one-compartment model with first-order absorption and elimination was used to describe the pharmacokinetics of tacrolimus in heart transplant patients. In the final model, the estimated apparent clearance (CL/F) and volume of distribution (V/F) were 532.5 L/h [12.20% interindividual variability, IIV] and 16.87 L (23.16% IIV), respectively. Albumin, postoperative time, and rs2242480 (CYP3A4*18B) gene polymorphisms were the significant covariates affecting CL/F, and creatinine clearance had significant effects on the V/F.
Conclusion
The population pharmacokinetic model of tacrolimus in heart transplant patients can better estimate the population and individual pharmacokinetic parameters of patients and can provide a reference for the design of individualized dosing regimens.



Similar content being viewed by others
References
Khush KK, Hsich E, Potena L, Cherikh WS, Chambers DC, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-eighth adult heart transplantation report—2021; focus on recipient characteristics. J Heart Lung Transplant. 2021;40(10):1035–49. https://doi.org/10.1016/j.healun.2021.07.015.
Scalea JR, Levi ST, Ally W, Brayman KL. Tacrolimus for the prevention and treatment of rejection of solid organ transplants. Expert Rev Clin Immunol. 2016;12(3):333–42. https://doi.org/10.1586/1744666x.2016.1123093.
Sikma MA, van Maarseveen EM, van de Graaf EA, Kirkels JH, Verhaar MC, Donker DW, et al. Pharmacokinetics and toxicity of tacrolimus early after heart and lung transplantation. Am J Transplant. 2015;15(9):2301–13. https://doi.org/10.1111/ajt.13309.
Regazzi MB, Rinaldi M, Molinaro M, Pellegrini C, Calvi M, Arbustini E, et al. Clinical pharmacokinetics of tacrolimus in heart transplant recipients. Ther Drug Monit. 1999;21(1):2–7. https://doi.org/10.1097/00007691-199902000-00002.
Sikma MA, Hunault CC, Van Maarseveen EM, Huitema ADR, Van de Graaf EA, Kirkels JH, et al. High variability of whole-blood tacrolimus pharmacokinetics early after thoracic organ transplantation. Eur J Drug Metab Pharmacokinet. 2020;45(1):123–34. https://doi.org/10.1007/s13318-019-00591-7.
Sikma MA, Hunault CC, Huitema ADR, De Lange DW, Van Maarseveen EM. Clinical pharmacokinetics and impact of hematocrit on monitoring and dosing of tacrolimus early after heart and lung transplantation. Clin Pharmacokinet. 2020;59(4):403–8. https://doi.org/10.1007/s40262-019-00846-1.
Brunet M, van Gelder T, Åsberg A, Haufroid V, Hesselink DA, Langman L, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41(3):261–307. https://doi.org/10.1097/ftd.0000000000000640.
Wang DD, Chen X, Fu M, Zheng QS, Xu H, Li ZP. Model extrapolation to a real-world dataset: evaluation of tacrolimus population pharmacokinetics and drug interaction in pediatric liver transplantation patients. Xenobiotica. 2020;50(4):371–9. https://doi.org/10.1080/00498254.2019.1631505.
Hannachi I, Ben Fredj N, Chadli Z, Ben Fadhel N, Ben Romdhane H, Touitou Y, et al. Effect of CYP3A4*22 and CYP3A4*1B but not CYP3A5*3 polymorphisms on tacrolimus pharmacokinetic model in Tunisian kidney transplant. Toxicol Appl Pharmacol. 2020;396: 115000. https://doi.org/10.1016/j.taap.2020.115000.
Rong Y, Mayo P, Ensom MHH, Kiang TKL. Population pharmacokinetics of mycophenolic acid co-administered with tacrolimus in corticosteroid-free adult kidney transplant patients. Clin Pharmacokinet. 2019;58(11):1483–95. https://doi.org/10.1007/s40262-019-00771-3.
Lu Z, Bonate P, Keirns J. Population pharmacokinetics of immediate- and prolonged-release tacrolimus formulations in liver, kidney and heart transplant recipients. Br J Clin Pharmacol. 2019;85(8):1692–703. https://doi.org/10.1111/bcp.13952.
Martial LC, Biewenga M, Ruijter BN, Keizer R, Swen JJ, van Hoek B, et al. Population pharmacokinetics and genetics of oral meltdose tacrolimus (Envarsus) in stable adult liver transplant recipients. Br J Clin Pharmacol. 2021;87(11):4262–72. https://doi.org/10.1111/bcp.14842.
Chen X, Wang DD, Xu H, Li ZP. Population pharmacokinetics and pharmacogenomics of tacrolimus in Chinese children receiving a liver transplant: initial dose recommendation. Transl Pediatr. 2020;9(5):576–86. https://doi.org/10.21037/tp-20-84.
Yang CL, Sheng CC, Liao GY, Su Y, Feng LJ, Xia Q, et al. Genetic polymorphisms in metabolic enzymes and transporters have no impact on mycophenolic acid pharmacokinetics in adult kidney transplant patients co-treated with tacrolimus: a population analysis. J Clin Pharm Ther. 2021;46(6):1564–75. https://doi.org/10.1111/jcpt.13488.
Zhang HJ, Li DY, Zhu HJ, Fang Y, Liu TS. Tacrolimus population pharmacokinetics according to CYP3A5 genotype and clinical factors in Chinese adult kidney transplant recipients. J Clin Pharm Ther. 2017;42(4):425–32. https://doi.org/10.1111/jcpt.12523.
Gong Y, Yang M, Sun Y, Li J, Lu Y, Li X. Population pharmacokinetic analysis of tacrolimus in Chinese cardiac transplant recipients. Eur J Hosp Pharm. 2020;27(e1):e12–8. https://doi.org/10.1136/ejhpharm-2018-001764.
Han Y, Zhou H, Cai J, Huang J, Zhang J, Shi SJ, et al. Prediction of tacrolimus dosage in the early period after heart transplantation: a population pharmacokinetic approach. Pharmacogenomics. 2019;20(1):21–35. https://doi.org/10.2217/pgs-2018-0116.
Rower JE, Stockmann C, Linakis MW, Kumar SS, Liu X, Korgenski EK, et al. Predicting tacrolimus concentrations in children receiving a heart transplant using a population pharmacokinetic model. BMJ Paediatr Open. 2017;1(1): e000147. https://doi.org/10.1136/bmjpo-2017-000147.
Kirubakaran R, Hennig S, Maslen B, Day RO, Carland JE, Stocker SL. Evaluation of published population pharmacokinetic models to inform tacrolimus dosing in adult heart transplant recipients. Br J Clin Pharmacol. 2022;88(4):1751–72. https://doi.org/10.1111/bcp.15091.
Li L, Li CJ, Zheng L, Zhang YJ, Jiang HX, Si-Tu B, et al. Tacrolimus dosing in Chinese renal transplant recipients: a population-based pharmacogenetics study. Eur J Clin Pharmacol. 2011;67(8):787–95. https://doi.org/10.1007/s00228-011-1010-y.
Wang P, Mao Y, Razo J, Zhou X, Wong ST, Patel S, et al. Using genetic and clinical factors to predict tacrolimus dose in renal transplant recipients. Pharmacogenomics. 2010;11(10):1389–402. https://doi.org/10.2217/pgs.10.105.
Kamdem LK, Streit F, Zanger UM, Brockmöller J, Oellerich M, Armstrong VW, et al. Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem. 2005;51(8):1374–81. https://doi.org/10.1373/clinchem.2005.050047.
Hebert MF. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev. 1997;27(2–3):201–14. https://doi.org/10.1016/s0169-409x(97)00043-4.
Liu S, Chen RX, Li J, Zhang Y, Wang XD, Fu Q, et al. The POR rs1057868-rs2868177 GC-GT diplotype is associated with high tacrolimus concentrations in early post-renal transplant recipients. Acta Pharmacol Sin. 2016;37(9):1251–8. https://doi.org/10.1038/aps.2016.77.
Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, et al. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281(26):17882–9. https://doi.org/10.1074/jbc.M601302200.
Lunde I, Bremer S, Midtvedt K, Mohebi B, Dahl M, Bergan S, et al. The influence of CYP3A, PPARA, and POR genetic variants on the pharmacokinetics of tacrolimus and cyclosporine in renal transplant recipients. Eur J Clin Pharmacol. 2014;70(6):685–93. https://doi.org/10.1007/s00228-014-1656-3.
Zhang X, Wang Z, Fan J, Liu G, Peng Z. Impact of interleukin-10 gene polymorphisms on tacrolimus dosing requirements in Chinese liver transplant patients during the early posttransplantation period. Eur J Clin Pharmacol. 2011;67(8):803–13. https://doi.org/10.1007/s00228-011-0993-8.
Seyhun Y, Ciftci HS, Kekik C, Karadeniz MS, Tefik T, Nane I, et al. Genetic association of interleukin-2, interleukin-4, interleukin-6, transforming growth factor-β, tumour necrosis factor-α and blood concentrations of calcineurin inhibitors in Turkish renal transplant patients. Int J Immunogenet. 2015;42(3):147–60. https://doi.org/10.1111/iji.12192.
Lu T, Zhu X, Xu S, Zhao M, Huang X, Wang Z, et al. Dosage optimization based on population pharmacokinetic analysis of tacrolimus in Chinese patients with nephrotic syndrome. Pharm Res. 2019;36(3):45. https://doi.org/10.1007/s11095-019-2579-6.
Toiyama Y, Okugawa Y, Tanaka K, Araki T, Uchida K, Hishida A, et al. A panel of methylated microRNA biomarkers for identifying high-risk patients with ulcerative colitis-associated colorectal cancer. Gastroenterology. 2017;153(6):1634-46.e8. https://doi.org/10.1053/j.gastro.2017.08.037.
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9(4):503–12. https://doi.org/10.1007/bf01060893.
Kirubakaran R, Stocker SL, Hennig S, Day RO, Carland JE. Population pharmacokinetic models of tacrolimus in adult transplant recipients: a systematic review. Clin Pharmacokinet. 2020;59(11):1357–92. https://doi.org/10.1007/s40262-020-00922-x.
Ogawa R, Stachnik JM, Echizen H. Clinical pharmacokinetics of drugs in patients with heart failure: an update (part 2, drugs administered orally). Clin Pharmacokinet. 2014;53(12):1083–114. https://doi.org/10.1007/s40262-014-0189-3.
Cheng F, Li Q, Wang J, Hu M, Zeng F, Wang Z, et al. Genetic polymorphisms affecting tacrolimus metabolism and the relationship to post-transplant outcomes in kidney transplant recipients. Pharmgenomics Pers Med. 2021;14:1463–74. https://doi.org/10.2147/pgpm.s337947.
Guy-Viterbo V, Scohy A, Verbeeck RK, Reding R, Wallemacq P, Musuamba FT. Population pharmacokinetic analysis of tacrolimus in the first year after pediatric liver transplantation. Eur J Clin Pharmacol. 2013;69(8):1533–42. https://doi.org/10.1007/s00228-013-1501-0.
Bergmann TK, Hennig S, Barraclough KA, Isbel NM, Staatz CE. Population pharmacokinetics of tacrolimus in adult kidney transplant patients: impact of CYP3A5 genotype on starting dose. Ther Drug Monit. 2014;36(1):62–70. https://doi.org/10.1097/FTD.0b013e31829f1ab8.
Chen B, Shi HQ, Liu XX, Zhang WX, Lu JQ, Xu BM, et al. Population pharmacokinetics and Bayesian estimation of tacrolimus exposure in Chinese liver transplant patients. J Clin Pharm Ther. 2017;42(6):679–88. https://doi.org/10.1111/jcpt.12599.
Jalil MH, Hawwa AF, McKiernan PJ, Shields MD, McElnay JC. Population pharmacokinetic and pharmacogenetic analysis of tacrolimus in paediatric liver transplant patients. Br J Clin Pharmacol. 2014;77(1):130–40. https://doi.org/10.1111/bcp.12174.
Genvigir FD, Salgado PC, Felipe CR, Luo EY, Alves C, Cerda A, et al. Influence of the CYP3A4/5 genetic score and ABCB1 polymorphisms on tacrolimus exposure and renal function in Brazilian kidney transplant patients. Pharmacogenet Genomics. 2016;26(10):462–72. https://doi.org/10.1097/fpc.0000000000000237.
Hesselink DA, van Schaik RH, van der Heiden IP, van der Werf M, Gregoor PJ, Lindemans J, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74(3):245–54. https://doi.org/10.1016/s0009-9236(03)00168-1.
Qiu XY, Jiao Z, Zhang M, Zhong LJ, Liang HQ, Ma CL, et al. Association of MDR1, CYP3A4*18B, and CYP3A5*3 polymorphisms with cyclosporine pharmacokinetics in Chinese renal transplant recipients. Eur J Clin Pharmacol. 2008;64(11):1069–84. https://doi.org/10.1007/s00228-008-0520-8.
Tamashiro EY, Felipe CR, Genvigir FDV, Rodrigues AC, Campos AB, Hirata RDC, et al. Influence of CYP3A4 and CYP3A5 polymorphisms on tacrolimus and sirolimus exposure in stable kidney transplant recipients. Drug Metab Pers Ther. 2017;32(2):89–95. https://doi.org/10.1515/dmpt-2016-0036.
Liu F, Ou YM, Yu AR, Xiong L, Xin HW. Long-term influence of CYP3A5, CYP3A4, ABCB1, and NR1I2 polymorphisms on tacrolimus concentration in Chinese renal transplant recipients. Genet Test Mol Biomarkers. 2017;21(11):663–73. https://doi.org/10.1089/gtmb.2017.0088.
Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623–53. https://doi.org/10.2165/00003088-200443100-00001.
Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914–56. https://doi.org/10.1016/j.healun.2010.05.034.
Hsu CY, Bates DW, Kuperman GJ, Curhan GC. Relationship between hematocrit and renal function in men and women. Kidney Int. 2001;59(2):725–31. https://doi.org/10.1046/j.1523-1755.2001.059002725.x.
Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90(2):154–66. https://doi.org/10.1016/j.cmpb.2007.12.002.
Yan-xia, Lu Qing-hong, Su Ke-hua, Wu Yu-peng, Ren Liang, Li Tian-yan, Zhou Wei, Lu (2015) A population pharmacokinetic study of tacrolimus in healthy Chinese volunteers and liver transplant patients. Acta Pharmacologica Sinica 36(2) 281-288 https://doi.org/10.1038/aps.2014.110
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by grants from the Natural Science Foundation of Fujian Province of China (grant no. 2018Y0037, 2021J01761), Fujian Medical Innovation Project (grant no. 2019-CX-19), and Startup Fund for scientific research, Fujian Medical University (grant no. 2019QH1269).
Conflict of interest
The authors report no conflicts of interest.
Ethics approval
This retrospective study involves human participants who were following the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Ethics Committee of Fujian Medical University Union Hospital (2020YF022-01) approved this study.
Consent to participate
Informed consent was obtained from the study participants before study commencement.
Consent to publish
Not applicable.
Author contribution
Conceptualization: Y.C., H.Q., and J.Z. Methodology: Y.C. and X.L. Data curation: Y.C., X.L., and J.C. Formal analysis and investigation: Y.C. and J.C. Writing: Y.C. Supervision: H.Q. and J.Z.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The coding for this study is available from the corresponding author upon reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, Y., Chen, J., Lin, X. et al. Population Pharmacokinetic Analysis for Model-Based Therapeutic Drug Monitoring of Tacrolimus in Chinese Han Heart Transplant Patients. Eur J Drug Metab Pharmacokinet 48, 89–100 (2023). https://doi.org/10.1007/s13318-022-00807-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00807-3