Abstract
Background and Objective
Gentamicin is commonly used in neonates, and it requires drug concentration monitoring. The objective of this study was to determine the extent of high trough (≥ 2 mg/l) and therapeutic peak serum gentamicin concentrations (5–12 mg/l) using our current gentamicin regimen and to adjust the dosing regimen accordingly and reassess.
Methods
This was a prospective cohort study of neonates, with normal renal function, who were prescribed gentamicin. Group 1: March 2014–July 2017—gentamicin intravenous (IV) 2.5 mg/kg given every 36 h if < 30 weeks gestational age (GA) and every 24 h if ≥ 30 weeks GA; Group 2: August 2019–February 2020—gentamicin IV 3.5 mg/kg given every 36 h if < 30 weeks GA and every 24 h if ≥ 30 weeks GA. We assessed the number of neonates with aberrant trough and peak serum gentamicin concentrations.
Results
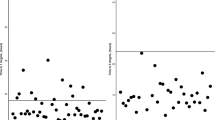
Forty-eight neonates < 30 weeks GA and 34 ≥ 30 weeks GA were given 2.5 mg/kg gentamicin. Eleven (23%) neonates < 30 weeks GA and four (13%) ≥ 30 weeks GA had subtherapeutic peak concentrations (< 5 mg/l); none had supratherapeutic (> 12 mg/l) or toxic trough concentrations (≥ 2 mg/l). Forty-four neonates < 30 weeks GA and 54 ≥ 30 weeks GA were given 3.5 mg/kg gentamicin. Eighty-four (86%) had non-toxic trough concentrations (< 2 mg/l). One (1%) < 30 weeks GA neonate had subtherapeutic (< 5 mg/l) and one (1%) neonate ≥ 30 weeks GA had supratherapeutic (> 12 mg/l) peak concentrations.
Conclusions
Gentamicin regimen of 2.5 mg/kg given every 36 h for neonates < 30 weeks GA and every 24 h for neonates ≥ 30 weeks GA was suboptimal at achieving therapeutic gentamicin peak. Increasing the dosage to 3.5 mg/kg achieved therapeutic peak concentrations in 98% and non-toxic trough concentrations in 86% of all neonates (prior to dose interval adjustment).
Similar content being viewed by others
References
Hagen I, Oymar K. Pharmacological differences between once daily and twice daily gentamicin dosage in newborns with suspected sepsis. Pharm World Sci. 2009;31:18–23.
Low YS, Tan SL, Wan AS. Extended-interval gentamicin dosing in achieving therapeutic concentrations in Malaysian Neonates. J Pediatr Pharmacol Ther. 2015;20:119–25.
Hitron AE, Yao S, Scarpace SB. Accuracy of empiric gentamicin dosing guidelines in neonates. J Pediatr Pharmacol Ther. 2010;15:264–73.
Lannigian R, Thomson A. Evaluation of 22 neonatal gentamicin dosage protocols using Bayesian approach. Paediatr Perinat Drug Ther. 2001;4:92–100.
Skopnik H, Wallraf R, Nies B, Troster K, Heimann G. Pharmacokinetics and antibacterial activity of daily gentamicin. Arch Dis Childhood. 1992;67:57–61.
Kadambari S, Heath PT, Sharland M, Lewis S, Nichols A, Turner MA. Variation in gentamicin and vancomycin dosage and monitoring in UK neonatal units. J Antimicrob Chemother. 2011;66:2647–50.
Davies MW. Gentamicin dosage in neonates. Infect Med. 2000;17:634.
Davies MW, Cartwright DW. Gentamicin dosage intervals. J Paediatr Child Health. 1999;35:412.
Rao SC, Ahmed M, Hagan R. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev. 2006;25:CD005091.
Fullas F, Padomek MT, Thieman CJ, Van Gorp AE. Comparative evaluation of six extended-interval gentamicin dosing regimens in preterm and full-term neonates. Am J Health-Syst Pharm. 2011;68:52–6.
Langlass TM, Mickle TR. Standard gentamicin dosage regimen in neonates. Am J Health-Syst Pharm. 1999;56:440–3.
Murphy JE, Austin ML, Frye RF. Evaluation of gentamicin pharmacokinetics and dosing protocols in 195 neonates. Am J Health-Syst Pharm. 1998;55:2280–8.
DiCenzo R, Forrest A, Slish JC, et al. A gentamicin pharmacokinetic population model and once-daily dosing algorithm for neonates. Pharmcotherapy. 2003;23:585–91.
Bauer LA. Aminoglycoside antibiotics. In: Bauer LA, editor. Clinical pharmacokinetics handbook. New York: The McGraw-Hill Companies; 2005. p. 55–7.
Hansen A, Forbes P, Arnold A, O’Rouke E. Once-daily gentamicin dosing for the preterm and term newborn: Proposal for a simple regimen that achieves target levels. J Perinat Med. 2003;23:635–9.
Hoff DS, Wilcox RA, Tollefson LM, Lipnik PG, Commers AR, Liu M. Pharmacokinetic outcomes of a simplified, weight-based, extended-interval gentamicin dosing protocol in critically ill neonates. Pharmacotherapy. 2009;29:1297–305.
Touw DJ, Westerman EM, Sprij AJ. Therapeutic drug monitoring of aminoglycosides in neonates. Clin Pharmacokinet. 2009;48(2):71–88.
Musiime GM, Seale AC, Moxon SG, Lawn JE. Risk of gentamicin toxicity in neonates treated for possible severe bacterial infection in low- and middle-income countries: systematic review. Trop Med Int Health. 2015;20(12):1593–606.
Kent A, Turner MA, Sharland M, Heath PT. Aminoglycoside toxicity in neonates: something to worry about? Expert Rev Anti Infect Ther. 2014;12(3):319–31.
Davies MW, Cartwright DW. Gentamicin dosage intervals in neonates: longer dosage interval—less toxicity. J Paediatr Child Health. 1998;34:577–80.
Feldman H, Guignard J-P. Plasma creatinine in the first month of life. Arch Dis Child. 1982;57:123–6.
Ahmed TA. Pharmacokinetics of drugs following IV bolus, IV infusion, and oral administration [Internet]. Intechopen; 2015 [cited 2019 Jul 9]. https://www.intechopen.com/books/basic-pharmacokinetic-concepts-and-some-clinical-applications/pharmacokinetics-of-drugs-following-iv-bolus-iv-infusion-and-oral-administration, https://doi.org/10.5772/61573
Beckman Coulter Inc. Chemistry Information Sheet 0151Gentamicin. https://www.beckmancoulter.com/wsrportal/techdocs?docname=/cis/A18492/%25%25/EN_GEN.pdf. Accessed 6 July 2021.
Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–9.
Nestaas E, Bangstad HJ, Sandvik L, Wathe KO. Aminoglycoside extended interval dosing in neonates is safe and effective: a meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2005;90:294–300.
Barclay ML, Begg EJ. Aminoglycoside adaptive resistance: importance for effective dosage regimens. Drugs. 2001;61:713–21.
Koren G. Therapeutic drug monitoring principles in the neonate. Clin Chem. 1997;43:222–7.
Gooding N, Elias-Jones A, Shenoy M. Gentamicin dosing in neonatal patients. Pharm World Sci. 2001;23:179–80.
Low YS, Tan SL, Wan ASL. Extended-interval gentamicin dosing in achieving therapeutic concentrations in Malaysian neonates. J Pediatric Pharmacol Ther. 2015;20(2):119–27.
García B, Barcia E, Pérez F, Molina IT. Population pharmacokinetics of gentamicin in preterm newborns. J Antimicrob Chemother. 2006;58(2):372–9.
Stolk LML, Degraeuwe PLJ, Nieman FHM, De Wolf MC, Boer AD. Population pharmacokinetics and relationship between demographic and clinical variables and pharmacokinetics of gentamicin in neonates. Ther Drug Monit. 2002;24:527–31.
Acknowledgements
Queensland Health Pathology Services and Ms Anje Van Aswegen and Ms Acacia Fairweather for assistance with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No source of funding.
Conflict of interest
No conflicts of interest.
Ethics approval
This study received ethical approval from the Royal Brisbane and Women’s Hospital Human Research Ethics Committee (reference no. HREC/13/QRBW/12), and all procedures in this study were in accordance with the 1964 Helsinki Declaration (and its amendments).
Consent to participate
The parent or carer of each infant gave written informed consent for their infant to participate in the study.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
MD, KW and DC contributed to the conception and design of this study; KOC and KW performed the statistical analysis and drafted the manuscript; PK, MD and DC critically reviewed the manuscript; MD and KW supervised the whole study process. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
O’Connor, K., Davies, M.W., Koorts, P. et al. Gentamicin Dosing in Neonates with Normal Renal Function: Trough and Peak Levels. Eur J Drug Metab Pharmacokinet 46, 677–684 (2021). https://doi.org/10.1007/s13318-021-00708-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-021-00708-x