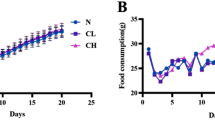
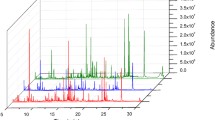
Abstract
Background and Objective
Ginseng is usually consumed as a dietary supplement for health care in the normal state or prescribed as a herbal medicine in pathologic conditions. Although metabolic studies of ginseng are commonly performed on healthy organisms, the metabolic characteristics in pathologic organisms remain unexplored. This study aimed to uncover the difference in intestinal metabolism of ginseng between normal and cyclophosphamide-induced immunosuppressed rats and further discuss the potential mechanisms involved.
Methods
Twelve Sprague-Dawley rats (6–8 weeks old) were randomly divided into two groups: the normal group (NG) and immunosuppressed group (ISG). Rats in the NG and ISG groups were intraperitoneally administered normal saline and cyclophosphamide injections (40 mg/kg) on the 1st, 2nd, 3rd and 10th days; on the 12th day, all rats were intragastrically administered ginseng water extract (900 mg/kg). The difference in intestinal metabolism of ginseng was compared using an ultra-high-performance liquid chromatography coupled with quadruple time-of-flight mass spectrometry-based metabolomics approach, and the diversities of gut microbiota were analyzed by 16S rRNA gene sequencing between the two groups.
Results
The intestinal metabolomic characteristics of ginseng were significantly different between the normal and immunosuppressed rats, with the ginsenoside F2 (F2), 20S-ginsenoside Rg3 (20(S)-Rg3), pseudo-ginsenoside Rt5 (Pseudo-Rt5), ginsenoside Rd (Rd), ginsenoside Rh1 (Rh1), 20S-ginsenoside Rg1 (20(S)-Rg1), ginsenoside compound K (CK), ginsenoside Rg2 (Rg2) and 20S-panaxatriol (S-PPT) more abundant in immunosuppressed ones (P < 0.05). Additionally, the composition of gut microbiota was remarkably altered in the two groups, with some specific bacterial communities such as Bacteroides spp., Eubacterium spp. and Lachnospiraceae_UCG-010 spp. increased and Bifidobacterium spp. decreased in immunosuppressed rats compared with normal ones.
Conclusion
The intestinal metabolism of ginseng in immunosuppressed rats was significantly different from that in normal ones, which might be partly attributed to the changes in the intensity of specific gut bacteria. The outcomes of this study could provide scientific data for rationalization of ginseng use as both a dietary supplement and herbal medicine.







Similar content being viewed by others
References
Zhu H, Long MH, Wu J, Wang MM, Li XY, Shen H, Xu JD, Zhou L, Fang ZJ, Luo Y, Li SL. Ginseng alleviates cyclophosphamide-induced hepatotoxicity via reversing disordered homeostasis of glutathione and bile acid. Sci Rep. 2015;5:17536.
Block KI, Mead MN. Immune system effects of echinacea, ginseng, and astragalus: a review. Integr Cancer Ther. 2003;2:247–67.
Qi LW, Wang CZ, Yuan CS. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011;28:467–95.
Ma B, Kan WL, Zhu H, Li SL, Lin G. Sulfur fumigation reducing systemic exposure of ginsenosides and weakening immunomodulatory activity of ginseng. J Ethnopharmacol. 2017;195:222–30.
Ru W, Wang D, Xu Y, He X, Sun YE, Qian L, Zhou X, Qin Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.). Drug Discov Ther. 2015;9:23–322.
Kim JH, Doo EH, Jeong M, Kim S, Lee YY, Yang J, Lee JS, Kim JH, Lee KW, Huh CS, Byun S. Enhancing immunomodulatory function of red ginseng through fermentation using Bifidobacterium animalis Subsp. lactis LT 19–2. Nutrients. 2019;11:1481.
Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 1998;50:1155–60.
Lee J, Lee E, Kim D, Lee J, Yoo J, Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol. 2009;122:143–8.
Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–71.
Zhu H, Shen H, Xu J, Xu JD, Zhu LY, Wu J, Chen HB, Li SL. Comparative study on intestinal metabolism and absorption in vivo of ginsenosides in sulphur-fumigated and non-fumigated ginseng by ultra performance liquid chromatography quadruple time-of-flight mass spectrometry based chemical profiling approach. Drug Test Anal. 2015;7:320–30.
Nicholson JK, Lindon JC. Systems biology: metabonomics. Nature. 2008;455:1054–6.
Li SL, Song JZ, Qiao CF, Zhou Y, Qian K, Lee KH, Xu HX. A novel strategy to rapidly explore potential chemical markers for the discrimination between raw and processed Radix Rehmanniae by UHPLC-TOFMS with multivariate statistical analysis. J Pharm Biomed Anal. 2010;51:812–23.
Kong M, Liu HH, Wu J, Shen MQ, Wang ZG, Duan SM, Zhang YB, Zhu H, Li SL. Effects of sulfur-fumigation on the pharmacokinetics, metabolites and analgesic activity of Radix Paeoniae Alba. J Ethnopharmacol. 2018;212:95–105.
Ju F, Zhang T. 16S rRNA gene high-throughput sequencing data mining of microbial diversity and interactions. Appl Microbiol Biotechnol. 2015;99:4119–29.
Vieira-Silva S, Falony G, Belda E, Nielsen T, Aron-Wisnewsky J, Chakaroun R, Forslund SK, Assmann K, Valles-Colomer M, Nguyen TTD, Proost S, Prifti E, Tremaroli V, Pons N, Le Chatelier E, Andreelli F, Bastard JP, Coelho LP, Galleron N, Hansen TH, Hulot JS, Lewinter C, Pedersen HK, Quinquis B, Rouault C, Roume H, Salem JE, Søndertoft NB, Touch S, Dumas ME, Ehrlich SD, Galan P, Gøtze JP, Hansen T, Holst JJ, Køber L, Letunic I, Nielsen J, Oppert JM, Stumvoll M, Vestergaard H, Zucker JD, Bork P, Pedersen O, Bäckhed F, Clément K, Raes J. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581:310–5.
Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, Zabari M, Brik RB, Kviatcovsky D, Zmora N, Cohen Y, Bar N, Levi I, Amar N, Mehlman T, Brandis A, Biton I, Kuperman Y, Tsoory M, Alfahel L, Harmelin A, Schwartz M, Israelson A, Arike L, Johansson MEV, Hansson GC, Gotkine M, Segal E, Elinav E. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572:474–80.
Zhou SS, Xu JD, Zhu H, Shen H, Xu J, Mao Q, Li SL, Yan R. Simultaneous determination of original, degraded ginsenosides and aglycones by ultra high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry for quantitative evaluation of Du-Shen-Tang, the decoction of ginseng. Molecules. 2014;19:4083–104.
Li SL, Lai SF, Song JZ, Qiao CF, Liu X, Zhou Y, Cai H, Cai BC, Xu HX. Decocting-induced chemical transformations and global quality of Du–Shen–Tang, the decoction of ginseng evaluated by UPLC-Q-TOF–MS/MS based chemical profiling approach. J Pharm Biomed Anal. 2010;53:946–57.
García-Villalba R, Selma MV, Espín JC, Tomás-Barberán FA. Identification of novel urolithin metabolites in human feces and urine after the intake of a pomegranate extract. J Agric Food Chem. 2019;67:11099–107.
Zhou G, Zhang Y, Li Y, Wang M, Li X. The metabolism of a natural product mogroside V, in healthy and type 2 diabetic rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1079:25–33.
Gan L, Ji J, Wang L, Li QY, Zhang CF, Wang CZ, Yuan CS. Identification of the metabolites in normal and AA rat plasma, urine and feces after oral administration of Daphne genkwa flavonoids by LC–Q-TOF–MS spectrometry. J Pharm Biomed Anal. 2020;177:112856.
Shin MS, Song JH, Choi P, Lee JH, Kim SY, Shin KS, Ham J, Kang KS. Stimulation of innate immune function by Panax ginseng after heat processing. J Agric Food Chem. 2018;66:4652–9.
Liu X, Zhang Z, Liu J, Wang Y, Zhou Q, Wang S, Wang X. Ginsenoside Rg3 improves cyclophosphamide-induced immunocompetence in Balb/c mice. Int Immunopharmacol. 2019;72:98–111.
Wang R, Zhang M, Hu S, Liu K, Tai Y, Tao J, Zhou W, Zhao Z, Wang Q, Wei W. Ginsenoside metabolite compound-K regulates macrophage function through inhibition of β-arrestin2. Biomed Pharmacother. 2019;115:108909.
Shen H, Zhang L, Xu J-D, Ding Y-F, Zhou J, Wu J, Zhang W, Mao Q, Liu L-F, Zhu H, Li S-L. Effect of sulfur-fumigation process on ginseng: metabolism and absorption evidences. J Ethnopharmacol. 2020;256:112799.
Kim DH. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J Ginseng Res. 2018;42:255–63.
Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84.
Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69:1510–9.
Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219–32.
Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74.
Thaiss CA, Levy M, Suez J, Elinav E. The interplay between the innate immune system and the microbiota. Curr Opin Immunol. 2014;26:41–8.
Shirani K, Hassani FV, Razavi-Azarkhiavi K, Heidari S, Zanjani BR, Karimi G. Phytotrapy of cyclophosphamide-induced immunosuppression. Environ Toxicol Pharmacol. 2015;39:1262–75.
Xu X, Zhang X. Effects of cyclophosphamide on immune system and gut microbiota in mice. Microbiol Res. 2015;171:97–106.
Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14:356–65.
Ying M, Yu Q, Zheng B, Wang H, Wang J, Chen S, Nie S, Xie M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr Polym. 2020;235:115957.
Sun Y, Liu Y, Ai C, Song S, Chen X. Caulerpa lentillifera polysaccharides enhance the immunostimulatory activity in immunosuppressed mice in correlation with modulating gut microbiota. Food Funct. 2019;10:4315–29.
Rajakovich LJ, Balskus EP. Metabolic functions of the human gut microbiota: the role of metalloenzymes. Nat Prod Rep. 2019;36:593–625.
Bae EA, Park SY, Kim DH. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481–5.
Yang L, Zou H, Gao Y, Luo J, Xie X, Meng W, Zhou H, Tan Z. Insights into gastrointestinal microbiota-generated ginsenoside metabolites and their bioactivities. Drug Metab Rev. 2020;52:125–38.
Kim K-A, Jung I-H, Park S-H, Ahn Y-T, Huh C-S, Kim D-H. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS One. 2013;8:e62409.
Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908–12.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by the National Key R&D Program of China (2019YFC1711000), National Natural Science Foundation of China (81873094 and 81503365), Independent Research Projects of Jiangsu Province Academy of Traditional Chinese Medicine (BM2018024-2019001 and BM2018024-2019009) and Natural Science Foundation of Jiangsu Province, China (BK20191504).
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
This study was approved by the Animal Ethics Committee of Jiangsu Province Academy of Traditional Chinese Medicine, Nanjing, PR China.
Author contributions
JHZ designed the study, performed the experiment, analyzed the results and drafted the manuscript; JDX, SSZ, XYZ, JZ, MK and QM performed the experiment partly; HZ and SLL acquired the funds, designed the study, analyzed the results and revised the manuscript.
Consent to participate
Not applicable.
Consent for publication
All authors approve the publication of this study.
Code availability
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, JH., Xu, JD., Zhou, SS. et al. Differences in Intestinal Metabolism of Ginseng Between Normal and Immunosuppressed Rats. Eur J Drug Metab Pharmacokinet 46, 93–104 (2021). https://doi.org/10.1007/s13318-020-00645-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-020-00645-1