Abstract
Background and Objective
Tacrolimus clearance (CL) is significantly altered according to recovery of liver function at an early stage after living donor liver transplantation (LDLT). In this study, we aimed to examine the impact of the change rate from postoperative day (POD) 1 in CL (ΔCL) of tacrolimus during continuous intravenous infusion (CIVI) on recipient recovery.
Methods
A tacrolimus population pharmacokinetic model on POD1 after LDLT was developed using Phoenix NLME 1.3. The CLPOD1 was calculated using the final model. The CLPOD4–7 was calculated by dividing total daily tacrolimus dose by the area under the concentration-time curve from 0 to 24 h.
Results
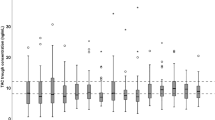
Data were obtained from 57 LDLT recipients, along with 540 points (177 points on POD1, 363 points on POD4–7) of tacrolimus whole blood concentrations at CIVI. The median tacrolimus CL decreased from POD1 to POD4 (from 2.73 to 1.40 L/h) and was then stable until POD7. Stepwise Cox proportional hazards regression analyses showed that the graft volume (GV)/standard liver volume (SLV) ratio (GV/SLV) and the tacrolimus ΔCLPOD6 were independent factors predicting early discharge (within 64 days median value) of recipients after LDLT [hazard ratio (HR) = 1.041, P = 0.001 and HR = 1.023, P = 0.004].
Conclusions
The tacrolimus ΔCL during CIVI immediately after LDLT in each recipient was a useful indicator for evaluation of recovery at an early stage after LDLT.




Similar content being viewed by others
References
Plosker GL, Foster RH. Tacrolimus: a further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs. 2000;59:323–89.
Scott LJ, McKeage K, Keam SJ, Plosker GL. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs. 2003;63:1247–97.
Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–53.
Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31:139–52.
Chen CL, Fan ST, Lee SG, Makuuchi M, Tanaka K. Living-donor liver transplantation: 12 years of experience in Asia. Transplantation. 2003;75:S6–S11.
Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53.
Haga J, Shimazu M, Wakabayashi G, et al. Liver regeneration in donors and adult recipients after living donor liver transplantation. Liver Transpl. 2008;14:1718–24.
Sugawara Y, Makuuchi M, Takayama T, et al. Small-for-size grafts in living-related liver transplantation. J Am Coll Surg. 2001;192:510–3.
Tucker ON, Heaton N. The 'small for size' liver syndrome. Curr Opin Crit Care. 2005;11:150–5.
Selzner M, Kashfi A, Cattral MS, et al. A graft to body weight ratio less than 0.8 does not exclude adult-to-adult right-lobe living donor liver transplantation. Liver Transpl. 2009;15:1776–822.
Eguchi S, Yanaga K, Sugiyama N, Okudaira S, Furui J, Kanematsu T. Relationship between portal venous flow and liver regeneration in patients after living donor right-lobe liver transplantation. Liver Transpl. 2003;9:547–51.
Park MY, Lee YJ, Rha SE, Oh SN, Byun JY, Kim DG. Correlation of portal venous velocity and portal venous flow with short-term graft regeneration in recipients of living donor liver transplants. Transpl Proc. 2008;40:1488–91.
Ganesh S, Almazroo OA, Tevar A, Humar A, Venkataramanan R. Drug metabolism, drug interactions, and drug-induced liver injury in living donor liver transplant patients. Clin Liver Dis. 2017;21:181–96.
Tanaka E, Inomata S, Yasuhara H. The clinical importance of conventional and quantitative liver function tests in liver transplantation. J Clin Pharm Ther. 2000;25:411–9.
Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–21.
Ferroni P, Martini F, D'Alessandro R, Magnapera A, Raparelli V, Scarno A, Davì G, Basili S, Guadagni F. In vivo platelet activation is responsible for enhanced vascular endothelial growth factor levels in hypertensive patients. Clin Chim Acta. 2008;388:33–7.
Ju MK, Chang HK, Kim HJ, Huh KH, Ahn HJ, Kim MS, Kim SI, Kim YS. Is the affinity column-mediated immunoassay method suitable as an alternative to the microparticle enzyme immunoassay method as a blood tacrolimus assay? Transpl Proc. 2008;40:3673–8.
Goldaracena N, Echeverri J, Selzner M. Small-for-size syndrome in live donor liver transplantation-Pathways of injury and therapeutic strategies. Clin Transpl. 2017;31:e12885. https://doi.org/10.1111/ctr.12885
Marcos A, Olzinski AT, Ham JM, Fisher RA, Posner MP. The interrelationship between portal and arterial blood flow after adult to adult living donor liver transplantation. Transplantation. 2000;70:1697–703.
Smyrniotis V, Kostopanagiotou G, Kondi A, et al. Hemodynamic interaction between portal vein and hepatic artery flow in small-for-size split liver transplantation. Transpl Int. 2002;15:355–60.
Yagi S, Iida T, Taniguchi K, et al. Impact of portal venous pressure on regeneration and graft damage after living-donor liver transplantation. Liver Transpl. 2005;11:68–75.
Morgan ET, Goralski KB, Piquette-Miller M, et al. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008;36:205–16.
Dickmann LJ, Patel SK, Wienkers LC, Slatter JG. Effects of interleukin 1β (IL-1β) and IL-1β/interleukin 6 (IL-6) combinations on drug metabolizing enzymes in human hepatocyte culture. Curr Drug Metab. 2012;13:930–7.
Jover R, Bort R, Gómez-Lechón MJ, Castell JV. Down-regulation of human CYP3A4 by the inflammatory signal interleukin-6: molecular mechanism and transcription factors involved. FASEB J. 2002;16:1799–801.
Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos. 2011;39:1415–22.
Friedman BH, Wolf JH, Wang L, et al. Serum cytokine profiles associated with early allograft dysfunction in patients undergoing liver transplantation. Liver Transpl. 2012;18:166–76.
Abdelaziz O, Attia H. Doppler ultrasonography in living donor liver transplantation recipients: intra- and post-operative vascular complications. World J Gastroenterol. 2016;22:6145–72.
Rodighiero V. Effects of liver disease on pharmacokinetics. An update Clin Pharmacokinet. 1999;37:399–43131.
Jusko WJ, Piekoszewski W, Klintmalm GB, et al. Pharmacokinetics of tacrolimus in liver transplant patients. Clin Pharmacol Ther. 1995;57:281–90.
Wallemacq PE, Furlan V, Möller A, et al. Pharmacokinetics of tacrolimus (FK506) in paediatric liver transplant recipients. Eur J Drug Metab Pharmacokinet. 1998;23:367–70.
Campagne O, Mager DE, Tornatore KM. Population pharmacokinetics of tacrolimus in transplant recipients: what did we learn about sources of interindividual variabilities? J Clin Pharmacol. 2019;59:309–25.
Fukatsu S, Yano I, Igarashi T, et al. Population pharmacokinetics of tacrolimus in adult recipients receiving living-donor liver transplantation. Eur J Clin Pharmacol. 2001;57:479–84.
Li M, Zhao Y, Humar A, Tevar AD, Hughes C, Venkataramanan R. Pharmacokinetics of drugs in adult living donor liver transplant patients: regulatory factors and observations based on studies in animals and humans. Expert Opin Drug Metab Toxicol. 2016;12:231–43.
Rojas LE, Herrero MJ, Bosó V, et al. Meta-analysis and systematic review of the effect of the donor and recipient CYP3A5 6986A%3eG genotype on tacrolimus dose requirements in liver transplantation. Pharmacogenet Genomics. 6986A;23(10):509–17.
Kato H, Usui M, Muraki Y, Okuda M, Nakatani K, Hayasaki A, et al. Intravenous administration of tacrolimus stabilizes control of blood concentration regardless of CYP3A5 polymorphism in living donor liver transplantation: comparison of intravenous infusion and oral administration in early postoperative period. Transpl Proc. 2018;50:2684–9.
Author information
Authors and Affiliations
Contributions
Conceptualization, TN; investigation, KI, NK, and TW; dose adjustment of tacrolimus, YT and YO; formal analysis, JN; writing—original draft preparation, JN; writing—review and editing; KH and TN. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
No funding was received to conduct this study.
Conflicts of interest
K.H. has received donation from Astellas Pharma Inc. The other authors have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Hirosaki University Graduate School of Medicine (project identification code: 2019–1021) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakagawa, J., Ishido, K., Tono, Y. et al. Relationship Between Change Rate of Tacrolimus Clearance During Continuous Intravenous Infusion and Recipient Recovery at an Early Stage After Living Donor Liver Transplantation. Eur J Drug Metab Pharmacokinet 45, 619–626 (2020). https://doi.org/10.1007/s13318-020-00628-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-020-00628-2