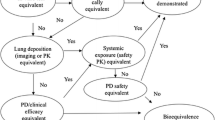
Abstract
The Japanese Ministry of Health, Labour and Welfare issued the basic principles for bioequivalence evaluations of generic dry powder inhaler (DPI) drug products in 2016. This document presents the recommendations of the methodology for the effective development of generic DPI drug products. Based on this document, the Pharmaceuticals and Medical Devices Agency (PMDA) advises the efficient development in the consultation meeting with generic companies. The PMDA generally requires the data of in vitro tests, pharmacokinetics studies, and clinical endpoint studies for generic development. In vitro tests play a critical role in the development of the generic versions because these tests are used to predict the efficacy and safety of other populations on whom clinical endpoint studies have not been conducted. We are aware that some points need further discussion, such as the recommendations for at least four groups of stages (group 1: the induction port and pre-separator, group 2: greater than 5 μm, group 3: ranging from 3 to 5 μm, group 4: ranging from 0.8 to 3 μm) for in vitro tests of the generic DPI products. This article shows the current understanding and recommendations with respect to in vitro tests, particularly for at least four groups of stages.

Similar content being viewed by others
References
US FDA. Draft guidance on albuterol sulfate. 2018. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM619932.pdf Accessed 19 Dec 2018.
US FDA. Draft guidance on mometasone furoate. 2016. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM495387.pdf Accessed 19 Dec 2018.
US FDA. Draft guidance on budesonide. 2012. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM319977.pdf Accessed 19 Dec 2018.
EMA. Guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease (COPD) in adults and for use in the treatment of asthma in children and adolescents. 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003504.pdf#search=‘EMA%2C?guideline?on?the?requirements?for?clinical%2C2009 Accessed 19 Dec 2018.
MHLW. Basic principles on the bioequivalence evaluation for the generic DPI drug products. 2016. https://www.pmda.go.jp/files/000210452.pdf#search=%27%E5%90%B8%E5%85%A5%E7%B2%89%E6%9C%AB%E5%89%A4?%E5%BE%8C%E7%99%BA%27 Accessed 21 Dec 2018 (In Japanese).
Kuribayashi R, Yamaguchi T, Sako H, Takishita T, Takagi K. Bioequivalence evaluations of generic dry powder inhaler drug products: similarities and differences between Japan, USA, and the European Union. Clin Pharmacokinet. 2017;56(3):225–33.
Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217–24.
Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(7):1103–7.
Hira D, Koide H, Nakamura S, Okada T, Ishizeki K, Yamaguchi M, Koshiyama S, Oguma T, Ito K, Funayama S, Komase Y, Morita SY, Nishiguchi K, Nakano Y, Terada T. Assessment of inhalation flow patterns of soft mist inhaler co-prescribed with dry powder inhaler using inspiratory flow meter for multi inhalation devices. PLoS One. 2018;13(2):e0193082.
Dunbar C, Mitchell J. Analysis of cascade impactor mass distributions. J Aerosol Med. 2005;18(4):439–51.
Aerosol consensus statement. Consensus conference on aerosol delivery. Chest. 1991;100(4):1106–9.
Tillie-Leblond I, Gosset P, Tonnel AB. Inflammatory events in severe acute asthma. Allergy. 2005;60(1):23–9.
Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–21.
Cukic V, Lovre V, Dragisic D, Ustamujic A. Asthma and chronic obstructive pulmonary disease (COPD)—differences and similarities. Mater Sociomed. 2012;24(2):100–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Ryosuke Kuribayashi, Aya Myoenzono, Kazunori Takagi, and Mitsue Hirota declare that they have no conflict of interest that might be relevant to the contents of this article.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official views of the Pharmaceuticals and Medical Devices Agency.
Rights and permissions
About this article
Cite this article
Kuribayashi, R., Myoenzono, A., Takagi, K. et al. Current Understanding of the Equivalence Evaluations for In Vitro Tests on Generic Dry Powder Inhaler Drug Products in Japan. Eur J Drug Metab Pharmacokinet 44, 743–745 (2019). https://doi.org/10.1007/s13318-019-00561-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-019-00561-z