Abstract
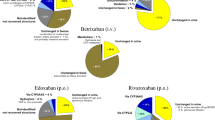
A recent survey on the use of direct oral anticoagulants (DOACs) revealed that 43% of patients with atrial fibrillation and renal impairment were potentially overdosed and had a hazard ratio for major bleeding of 2.19. In this review, we analyse and discuss the effect of renal failure on the pharmacokinetics and pharmacodynamics of DOACs and of strategies proposed to adjust doses according to the level of renal dysfunction. The pharmacokinetic characteristics of available DOACs (dabigatran, rivaroxaban, apixaban, edoxaban, betrixaban) differ substantially as regards oral bioavailability, plasma protein binding and the relative involvement of renal and non-renal elimination. In this respect, 80% of dabigatran is excreted as an unchanged drug in urine, whereas edoxaban, rivaroxaban, apixaban and betrixiban are excreted unchanged by, respectively, 50, 33, 27 and 11% of the dose. Therefore, drug exposure (the area under the concentration–time curve, AUC) is expected to increase to differing extents, depending on the residual renal function and the contribution of the kidneys to the excretion of each drug. Our analysis found that the increased AUC in patients with severe renal dysfunction was greater than expected in the case of dabigatran, betrixaban and rivaroxaban, indicating that other pharmacokinetic parameters may be altered besides renal clearance. Although DAOC pharmacodynamics do not seem to be altered by renal diseases (the correlation between plasma levels and anticoagulant effects overlaps that of healthy subjects), renal failure per se is associated with an increased risk of bleeding and thromboembolism. Guidelines on dose adjustments in patients with renal dysfunction have been published by three National Drug Agencies (FDA, EMA, HC), but many of their items do not match one another, reflecting our substantial paucity of knowledge in advanced renal failure. Routine monitoring of DOAC anticoagulant effects or plasma concentrations is not recommended, since no validated therapeutic ranges have been established. However, this approach may be useful in emergency situations such as bleeding or thrombotic events, urgent surgery, pharmacokinetic interactions, etc. We conclude that more experimental work is needed to improve our knowledge of DOAC pharmacology in renal failure and to provide clinicians with valid tools to adjust therapy.



Similar content being viewed by others
References
Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69(23):2779–90. https://doi.org/10.1016/j.jacc.2017.03.600.
Tucker GT. Measurement of the renal clearance of drugs. Br J Clin Pharmacol. 1981;12(6):761–70.
Nolin TD, Naud J, Leblond FA, Pichette V. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther. 2008;83(6):898–903. https://doi.org/10.1038/clpt.2008.59.
Dreisbach AW. The influence of chronic renal failure on drug metabolism and transport. Clin Pharmacol Ther. 2009;86(5):553–6. https://doi.org/10.1038/clpt.2009.163.
Nolin TD, Unruh ML. Clinical relevance of impaired nonrenal drug clearance in ESRD. Semin Dial. 2010;23(5):482–5.
Naud J, Nolin TD, Leblond FA, Pichette V. Current understanding of drug disposition in kidney disease. J Clin Pharmacol. 2012;52(1 Suppl):10S–22S. https://doi.org/10.1177/0091270011413588.
Tan ML, Yoshida K, Zhao P, Zhang L, Nolin TD, Piquette-Miller M, et al. Effect of chronic kidney disease on nonrenal elimination pathways: a systematic assessment of CYP1A2, CYP2C8, CYP2C9, CYP2C19, and OATP. Clin Pharmacol Ther. 2018;103(5):854–67. https://doi.org/10.1002/cpt.807.
Yoshida K, Sun B, Zhang L, Zhao P, Abernethy DR, Nolin TD, et al. Systematic and quantitative assessment of the effect of chronic kidney disease on CYP2D6 and CYP3A4/5. Clin Pharmacol Ther. 2016;100(1):75–87. https://doi.org/10.1002/cpt.337.
Bjornsson TD. Nomogram for drug dosage in patients with renal failure. Clin Pharmacokinet. 1986;11(2):164–70.
Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol. 2009;65(8):757–73. https://doi.org/10.1007/s00228-009-0678-8.
Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47(5):285–95.
Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36(2):386–99.
Ebner T, Wagner K, Wienen W. Dabigatran acylglucuronide, the major human metabolite of dabigatran: in vitro formation, stability, and pharmacological activity. Drug Metab Dispos. 2010;38(9):1567–75. https://doi.org/10.1124/dmd.110.033696.
Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol. 2012;26(1):27–32. https://doi.org/10.1111/j.1472-8206.2011.00981.x.
Weinz C, Schwarz T, Kubitza D, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos. 2009;37(5):1056–64. https://doi.org/10.1124/dmd.108.025569.
Byon W, Sweeney K, Frost C, Boyd RA. Population pharmacokinetics, pharmacodynamics, and exploratory exposure-response analyses of apixaban in subjects treated for venous thromboembolism. CPT Pharmacometrics Syst Pharmacol. 2017;6(5):340–9. https://doi.org/10.1002/psp4.12184.
Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37(1):74–81. https://doi.org/10.1124/dmd.108.023143.
Parasrampuria DA, Truitt KE. Pharmacokinetics and pharmacodynamics of edoxaban, a non-vitamin K antagonist oral anticoagulant that Inhibits clotting factor Xa. Clin Pharmacokinet. 2016;55(6):641–55. https://doi.org/10.1007/s40262-015-0342-7.
Bathala MS, Masumoto H, Oguma T, He L, Lowrie C, Mendell J. Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos. 2012;40(12):2250–5. https://doi.org/10.1124/dmd.112.046888.
BEVYXXA prescribing information (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208383s000lbl.pdf. Accessed 4 July 2018.
Betrixaban. Clinical pharmacology and biopharmaceutics review(s). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208383Orig1s000ClinPharmR.pdf. Accessed 4 July 2018.
Remko M. Molecular structure, lipophilicity solubility, absorption, and polar surface area of new anticoagulant agents. J Mol Struct Theochem. 2009;916:76–85. https://doi.org/10.1016/j.theochem.2009.09.011.
Remko M, Remková A, Broer R. Theoretical study of molecular structure and physicochemical properties of novel factor xa inhibitors and dual factor Xa and factor IIa inhibitors. Molecules. 2016;21:185. https://doi.org/10.3390/molecules21020185.
Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49(4):259–68. https://doi.org/10.2165/11318170-000000000-00000.
Kubitza D, Becka M, Mueck W, Halabi A, Maatouk H, Klause N, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol. 2010;70(5):703–12. https://doi.org/10.1111/j.1365-2125.2010.03753.x.
Chang M, Yu Z, Shenker A, Wang J, Pursley J, Byon W, et al. Effect of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of apixaban. J Clin Pharmacol. 2016;56(5):637–45. https://doi.org/10.1002/jcph.633.
Wang X, Tirucherai G, Marbury TC, Wang J, Chang M, Zhang D, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56(5):628–66. https://doi.org/10.1002/jcph.628.
Parasrampuria DA, Marbury T, Matsushima N, Chen S, Wickremasingha PK, He L, et al. Pharmacokinetics, safety, and tolerability of edoxaban in end-stage renal disease subjects undergoing haemodialysis. Thromb Haemost. 2015;113(4):719–27. https://doi.org/10.1160/TH14-06-0547.
Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76(3):455–66. https://doi.org/10.1111/bcp.12075.
Mendell J, Zahir H, Matsushima N, Noveck R, Lee F, Chen S, et al. Drug-drug interaction studies of cardiovascular drugs involving P-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor Xa inhibitor. Am J Cardiovasc Drugs. 2013;13(5):331–42. https://doi.org/10.1007/s40256-013-0029-0.
Strid H, Simrén M, Stotzer PO, Abrahamsson H, Björnsson ES. Delay in gastric emptying in patients with chronic renal failure. Scand J Gastroenterol. 2004;39:516–20.
Watanabe H, Hiraishi H, Ishida M, Kazama JJ, Terano AJ. Pathophysiology of gastric acid secretion in patients with chronic renal failure:influence of Helicobacter pylori infection. Intern Med. 2003;254:439–46.
Osborne R, Joel S, Grebenik K, Trew D, Slevin M. The pharmacokinetics of morphine and morphine glucuronides in kidney failure. Clin Pharmacol Ther. 1993;54(2):158–67.
Barnes KJ, Rowland A, Polasek TM, Miners JO. Inhibition of human drug-metabolising cytochrome P450 and UDP-glucuronosyltransferase enzyme activities in vitro by uremic toxins. Eur J Clin Pharmacol. 2014;70(9):1097–106. https://doi.org/10.1007/s00228-014-1709-7.
Frost CE, Byon W, Song Y, Wang J, Schuster AE, Boyd RA, et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br J Clin Pharmacol. 2015;79(5):838–46. https://doi.org/10.1111/bcp.12541.
Khadzhynov D, Wagner F, Formella S, Wiegert E, Moschetti V, Slowinski T, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost. 2013;109(4):596–605. https://doi.org/10.1160/TH12-08-0573.
Dias C, Moore KT, Murphy J, Ariyawansa J, Smith W, Mills RM, et al. Pharmacokinetics, pharmacodynamics, and safety of single-dose rivaroxaban in chronic hemodialysis. Am J Nephrol. 2016;43(4):229–36. https://doi.org/10.1159/000445328.
Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28(7):2241–8. https://doi.org/10.1681/ASN.2016090980.
Koretsune Y, Yamashita T, Kimura T, Fukuzawa M, Abe K, Yasaka M. Short-term safety and plasma concentrations of edoxaban in Japanese patients with non-valvular atrial fibrillation and severe renal impairment. Circ J. 2015;79(7):1486–95. https://doi.org/10.1253/circj.CJ-14-0942.
Del-Carpio Munoz F, Gharacholou SM, Munger TM, Friedman PA, Asirvatham SJ, Packer DL, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117(1):69–75. https://doi.org/10.1016/j.amjcard.2015.09.046.
Zou R, Tao J, Shi W, Yang M, Li H, Lin X, et al. Meta-analysis of safety and efficacy for direct oral anticoagulation treatment of non-valvular atrial fibrillation in relation to renal function. Thromb Res. 2017;160:41–50. https://doi.org/10.1016/j.thromres.2017.10.013.
Fanikos J, Burnett AE, Mahan CE, Dobesh PP. Renal function considerations for stroke prevention in atrial fibrillation. Am J Med. 2017;130(9):1015–23. https://doi.org/10.1016/j.amjmed.2017.04.015.
Andò G, Capranzano P. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with chronic kidney disease: a systematic review and network meta-analysis. Int J Cardiol. 2017;231:162–9. https://doi.org/10.1016/j.ijcard.2016.11.303.
Nielsen PB, Lane DA, Rasmussen LH, Lip GY, Larsen TB. Renal function and non-vitamin K oral anticoagulants in comparison with warfarin on safety and efficacy outcomes in atrial fibrillation patients: a systemic review and meta-regression analysis. Clin Res Cardiol. 2015;104(5):418–29. https://doi.org/10.1007/s00392-014-0797-9.
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–93. https://doi.org/10.1093/eurheartj/ehy136.
Heidenreich PA, Solis P, Mark Estes NA 3rd, Fonarow GC, Jurgens CY, Marine JE, et al. Clinical performance and quality measures for adults with atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circ Cardiovasc Qual Outcomes. 2016;9(4):443–88. https://doi.org/10.1161/HCQ.0000000000000018.
Macle L, Cairns J, Leblanc K, Tsang T, Skanes A, Cox JL, et al. CCS Atrial Fibrillation Guidelines Committee. 2016 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2016;32(10):1170–85. https://doi.org/10.1016/j.cjca.2016.07.591.
Hariharan S, Madabushi R. Clinical pharmacology basis of deriving dosing recommendations for dabigatran in patients with severe renal impairment. J Clin Pharmacol. 2012;52(1 Suppl):119S–25S. https://doi.org/10.1177/0091270011415527.
Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY trial (randomized evaluation of long-term anticoagulation therapy). J Am Coll Cardiol. 2014;63(4):321–8. https://doi.org/10.1016/j.jacc.2013.07.104.
Ruff CT, Giugliano RP, Braunwald E, Morrow DA, Murphy SA, Kuder JF, et al. Association between edoxaban dose, concentration, anti-factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48trial. Lancet. 2015;385:2288–95. https://doi.org/10.1016/S0140-6736(14)61943-7.
Song S, Kang D, Halim AB, Miller R. Population pharmacokinetic-pharmacodynamic modeling analysis of intrinsic FXa and bleeding from edoxaban treatment. J Clin Pharmacol. 2014;54(8):910–6. https://doi.org/10.1002/jcph.306.
Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest. 2017;151(1):127–38. https://doi.org/10.1016/j.chest.2016.08.1462.
Levy JH, Douketis J, Weitz JI. Reversal agents for non-vitamin K antagonist oral anticoagulants. Nat Rev Cardiol. 2018;15(5):273–81. https://doi.org/10.1038/nrcardio.2017.223.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This review was founded by the University of Padova, Italy (“fondi DOR”, 2017).
Conflict of interest
The author declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Padrini, R. Clinical Pharmacokinetics and Pharmacodynamics of Direct Oral Anticoagulants in Patients with Renal Failure. Eur J Drug Metab Pharmacokinet 44, 1–12 (2019). https://doi.org/10.1007/s13318-018-0501-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-018-0501-y