Abstract
Background and Objectives
Finerenone is a selective, non-steroidal mineralocorticoid receptor antagonist. In vivo and in vitro studies were performed to assess absolute bioavailability of finerenone, the effect of metabolic enzyme inhibitors on the pharmacokinetics of finerenone and its metabolites, the quantitative contribution of the involved enzymes cytochrome P450 (CYP) 3A4 and CYP2C8 and the relevance of gut wall versus liver metabolism.
Methods
The pharmacokinetics, safety and tolerability of finerenone (1.25–10 mg orally or 0.25–1.0 mg intravenously) were evaluated in healthy male volunteers in four crossover studies. Absolute bioavailability was assessed in volunteers receiving finerenone orally and by intravenous infusion (n = 15) and the effects of erythromycin (n = 15), verapamil (n = 13) and gemfibrozil (n = 16) on finerenone pharmacokinetics were investigated. Finerenone was also incubated with cryopreserved human hepatocytes in vitro in the presence of erythromycin, verapamil or gemfibrozil.
Results
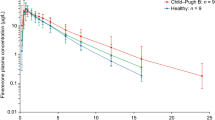
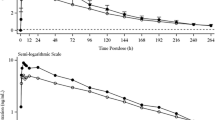
Finerenone absolute bioavailability was 43.5% due to first-pass metabolism in the gut wall and liver. The geometric mean AUC0–∞ ratios of finerenone (drug + inhibitor/drug alone) were 3.48, 2.70 and 1.10 with erythromycin, verapamil and gemfibrozil, respectively. The contribution ratio of CYP3A4 to the metabolic clearance of finerenone derived from these values was 0.88–0.89 and was consistent with estimations based on in vitro data, with the remaining metabolic clearance due to CYP2C8 involvement.
Conclusion
Finerenone is predominantly metabolized by CYP3A4 in the gut wall and liver. Increases in systemic exposure upon concomitant administration of inhibitors of this isoenzyme are predictable and consistent with in vitro data. Inhibition of CYP2C8, the second involved metabolic enzyme, has no relevant effect on finerenone in vivo.




Similar content being viewed by others
References
Bauersachs J, Jaisser F, Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension. 2015;65(2):257–63. https://doi.org/10.1161/hypertensionaha.114.04488.
Schwenk MH, Hirsch JS, Bomback AS. Aldosterone blockade in CKD: emphasis on pharmacology. Adv Chronic Kidney Dis. 2015;22(2):123–32. https://doi.org/10.1053/j.ackd.2014.08.003.
Kolkhof P, Bärfacker L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234(1):T125–40. https://doi.org/10.1530/joe-16-0600.
Kolkhof P, Jaisser F, Kim SY, Filippatos G, Nowack C, Pitt B. Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: comparison at bench and bedside. Handb Exp Pharmacol. 2017;243:271–305. https://doi.org/10.1007/164_2016_76.
Bärfacker L, Kuhl A, Hillisch A, Grosser R, Figueroa-Perez S, Heckroth H, et al. Discovery of BAY 94-8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem. 2012;7(8):1385–403. https://doi.org/10.1002/cmdc.201200081.
Kolkhof P, Nowack C, Eitner F. Nonsteroidal antagonists of the mineralocorticoid receptor. Curr Opin Nephrol Hypertens. 2015;24(5):417–24. https://doi.org/10.1097/mnh.0000000000000147.
Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Barfacker L, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69–78. https://doi.org/10.1097/fjc.0000000000000091.
Kolkhof P, Borden SA. Molecular pharmacology of the mineralocorticoid receptor: prospects for novel therapeutics. Mol Cell Endocrinol. 2012;350(2):310–7. https://doi.org/10.1016/j.mce.2011.06.025.
Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884–94. https://doi.org/10.1001/jama.2015.10081.
Filippatos G, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37(27):2105–14. https://doi.org/10.1093/eurheartj/ehw132.
Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34(31):2453–63. https://doi.org/10.1093/eurheartj/eht187.
Lentini S, Heinig R, Kimmeskamp-Kirschbaum N, Wensing G. Pharmacokinetics, safety and tolerability of the novel, selective mineralocorticoid receptor antagonist finerenone—results from first-in-man and relative bioavailability studies. Fundam Clin Pharmacol. 2016;30(2):172–84. https://doi.org/10.1111/fcp.12170.
Heinig R, Kimmeskamp-Kirschbaum N, Halabi A, Lentini S. Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (BAY 94-8862) in individuals with renal impairment. Clin Pharmacol Drug Dev. 2016;5(6):488–501. https://doi.org/10.1002/cpdd.263.
Gerisch M, Heinig R, Engelen A, Lang D, Kolkhof P, Platzek J et al. Biotransformation of finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist, in dogs, rats, and humans in vivo and in vitro. In preparation.
Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94(9):1140–6. https://doi.org/10.1016/j.amjcard.2004.07.080.
Yang QJ, Fan J, Chen S, Liu L, Sun H, Pang KS. Metabolite kinetics: the segregated flow model for intestinal and whole body physiologically based pharmacokinetic modeling to describe intestinal and hepatic glucuronidation of morphine in rats in vivo. Drug Metab Dispos. 2016;44(7):1123–38. https://doi.org/10.1124/dmd.116.069542.
Ohno Y, Hisaka A, Suzuki H. General framework for the quantitative prediction of CYP3A4-mediated oral drug interactions based on the AUC increase by coadministration of standard drugs. Clin Pharmacokinet. 2007;46(8):681–96. https://doi.org/10.2165/00003088-200746080-00005.
Loue C, Tod M. Reliability and extension of quantitative prediction of CYP3A4-mediated drug interactions based on clinical data. AAPS J. 2014;16(6):1309–20. https://doi.org/10.1208/s12248-014-9663-y.
Gertz M, Harrison A, Houston JB, Galetin A. Prediction of human intestinal first-pass metabolism of 25 CYP3A substrates from in vitro clearance and permeability data. Drug Metab Dispos. 2010;38(7):1147–58. https://doi.org/10.1124/dmd.110.032649.
Okudaira T, Kotegawa T, Imai H, Tsutsumi K, Nakano S, Ohashi K. Effect of the treatment period with erythromycin on cytochrome P450 3A activity in humans. J Clin Pharmacol. 2007;47(7):871–6. https://doi.org/10.1177/0091270007302562.
Hla KK, Henry JA, Latham AN. Pharmacokinetics and pharmacodynamics of two formulations of verapamil. Br J Clin Pharmacol. 1987;24(5):661–4.
Honkalammi J, Niemi M, Neuvonen PJ, Backman JT. Gemfibrozil is a strong inactivator of CYP2C8 in very small multiple doses. Clin Pharmacol Ther. 2012;91(5):846–55. https://doi.org/10.1038/clpt.2011.313.
Honkalammi J, Niemi M, Neuvonen PJ, Backman JT. Mechanism-based inactivation of CYP2C8 by gemfibrozil occurs rapidly in humans. Clin Pharmacol Ther. 2011;89(4):579–86. https://doi.org/10.1038/clpt.2010.358.
Floyd JS, Kaspera R, Marciante KD, Weiss NS, Heckbert SR, Lumley T, et al. A screening study of drug-drug interactions in cerivastatin users: an adverse effect of clopidogrel. Clin Pharmacol Ther. 2012;91(5):896–904. https://doi.org/10.1038/clpt.2011.295.
Walsky RL, Gaman EA, Obach RS. Examination of 209 drugs for inhibition of cytochrome P450 2C8. J Clin Pharmacol. 2005;45(1):68–78. https://doi.org/10.1177/0091270004270642.
Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos. 2006;34(5):880–6. https://doi.org/10.1124/dmd.105.008672.
Acknowledgements
The authors would like to acknowledge the investigators responsible for the different studies: Dr. Michael Leidig (CRS Mönchengladbach) for his key role in the absolute bioavailability study, Dr. Angela Kentrat (ClinPharmCologne), Manuela Casjens (CRS Berlin) and Dr. Sybille Baumann (CRS Mannheim) for their valuable contributions to the drug–drug interaction in vivo studies with erythromycin, verapamil and gemfibrozil, respectively. Dr. Gabriele Rohde of Bayer AG (Wuppertal, Germany) was responsible for bioanalyses. Medical writing assistance, funded by Bayer AG, was provided by Dr. Nicolas Bertheleme of Oxford PharmaGenesis, Oxford, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Research was funded by the sponsor, Bayer AG. Bayer AG agreed to the publication of the present data.
Conflict of interest
RH, MG, AE and JN are employees of Bayer AG. SL is an employee of Chrestos Concept GmbH & Co. KG, which received funding for this analysis from Bayer AG. In addition, RH, MG, AE and JN have stock in Bayer AG, but are not paid in stock or stock options.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all participants before study commencement.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Heinig, R., Gerisch, M., Engelen, A. et al. Pharmacokinetics of the Novel, Selective, Non-steroidal Mineralocorticoid Receptor Antagonist Finerenone in Healthy Volunteers: Results from an Absolute Bioavailability Study and Drug–Drug Interaction Studies In Vitro and In Vivo. Eur J Drug Metab Pharmacokinet 43, 715–727 (2018). https://doi.org/10.1007/s13318-018-0483-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-018-0483-9