Abstract
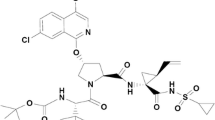
Elbasvir and grazoprevir, in a fixed-dose combination of 50 and 100 mg, respectively, have received approval to be administered orally once daily, with or without ribavirin, for the treatment of chronic hepatitis C virus (HCV) infections. The absorption characteristics of elbasvir and grazoprevir have been adequately summarized, although differences were observed for grazoprevir (e.g., increased exposure at steady state in patients), but not elbasvir, between healthy and HCV-infected subjects. Inconsistencies with respect to absorption were also reported on the effects of food or acid reducers (famotidine or pantoprazole) in the literature. Many distribution characteristics of elbasvir and grazoprevir have been obtained from in vitro models, using incubation conditions that were in many cases inconsistent with normal physiological conditions in humans. Elbasvir and grazoprevir appear to undergo modest hepatic metabolism and are excreted primarily unchanged (~ 80% parent drug found) in feces. Both elbasvir and grazoprevir are substrates of cytochrome P450 (CYP) 3A4 enzyme, but mechanistic experiments were lacking to demonstrate the role of other enzymes and the precise relative % contribution of CYP3A4. The pharmacokinetics of elbasvir and grazoprevir have been characterized in various special populations (elderly, male vs. female, Caucasian vs. Asian, renal impairment, and hepatic impairment). Other than moderate and severe hepatic impairment where administrations are contraindicated, no dose adjustments are required for both drugs in these special patient populations. The available drug–drug interaction data provided some consistency between in vitro and in vivo observations and, in some instances, can provide predictions of likely clinically relevant scenarios.
Similar content being viewed by others
References
Karaoui LR, Mansour H, Chahine EB. Elbasvir-grazoprevir: a new direct-acting antiviral combination for hepatitis C. Am J Health Syst Pharm. 2017;74:1533–40.
Majumdar A, Kitson MT, Roberts SK. Systematic review: current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol Ther. 2016;43:1276–92.
Myers RP, Shah H, Burak KW, Cooper C, Feld JJ. An update on the management of chronic hepatitis C: 2015 Consensus guidelines from the Canadian Association for the Study of the Liver. Can J Gastroenterol Hepatol. 2015;29:19–34.
Al-Salama ZT, Deeks ED. Elbasvir/Grazoprevir: a review in chronic HCV genotypes 1 and 4. Drugs. 2017;77:911–21.
Sulejmani N, Jafri SM, Gordon SC. Pharmacodynamics and pharmacokinetics of elbasvir and grazoprevir in the treatment of hepatitis C. Expert Opin Drug Metab Toxicol. 2016;12:353–61.
Alric L, Bonnet D. Grazoprevir + elbasvir for the treatment of hepatitis C virus infection. Expert Opin Pharmacother. 2016;17:735–42.
Lawitz E, Gane E, Pearlman B, Tam E, Ghesquiere W, Guyader D, Alric L, Bronowicki JP, Lester L, Sievert W, Ghalib R, Balart L, Sund F, Lagging M, Dutko F, Shaughnessy M, Hwang P, Howe AY, Wahl J, Robertson M, Barr E, Haber B. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385:1075–86.
Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, Brown DD, Wan S, DiNubile MJ, Nguyen BY, Robertson MN, Wahl J, Barr E, Butterton JR. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med. 2015;163:1–13.
Sulkowski M, Hezode C, Gerstoft J, Vierling JM, Mallolas J, Pol S, Kugelmas M, Murillo A, Weis N, Nahass R, Shibolet O, Serfaty L, Bourliere M, DeJesus E, Zuckerman E, Dutko F, Shaughnessy M, Hwang P, Howe AY, Wahl J, Robertson M, Barr E, Haber B. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385:1087–97.
Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, Matthews GV, Saag MS, Zamor PJ, Orkin C, Gress J, Klopfer S, Shaughnessy M, Wahl J, Nguyen BY, Barr E, Platt HL, Robertson MN, Sulkowski M. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV. 2015;2:e319–27.
Buti M, Gordon SC, Zuckerman E, Lawitz E, Calleja JL, Hofer H, Gilbert C, Palcza J, Howe AY, DiNubile MJ, Robertson MN, Wahl J, Barr E, Forns X. Grazoprevir, elbasvir, and ribavirin for chronic hepatitis C virus genotype 1 infection after failure of pegylated interferon and ribavirin with an earlier-generation protease inhibitor: final 24-week results from C-SALVAGE. Clin Infect Dis. 2016;62:32–6.
Forns X, Gordon SC, Zuckerman E, Lawitz E, Calleja JL, Hofer H, Gilbert C, Palcza J, Howe AY, DiNubile MJ, Robertson MN, Wahl J, Barr E, Buti M. Grazoprevir and elbasvir plus ribavirin for chronic HCV genotype-1 infection after failure of combination therapy containing a direct-acting antiviral agent. J Hepatol. 2015;63:564–72.
Bruchfeld A, Roth D, Martin P, Nelson DR, Pol S, Londono MC, Monsour H Jr, Silva M, Hwang P, Arduino JM, Robertson M, Nguyen BY, Wahl J, Barr E, Greaves W. Elbasvir plus grazoprevir in patients with hepatitis C virus infection and stage 4-5 chronic kidney disease: clinical, virological, and health-related quality-of-life outcomes from a phase 3, multicentre, randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2017;2:585–94.
Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr, Martin P, Pol S, Londono MC, Hassanein T, Zamor PJ, Zuckerman E, Wan S, Jackson B, Nguyen BY, Robertson M, Barr E, Wahl J, Greaves W. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–45.
European Medicines Agency AR. Zepatier (elbasvir/grazoprevir) Assessment Report. European Medicines Agency. 2016;EMEA/H/C/004126/0000:1-151. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004126/WC500211237.pdf. Accessed 11 Jan 2018.
Center for Drug Evaluation and Research Clinical Pharmacology and Biopharmaceutics Review. 505(b)(1) New Drug Application, Priority Review (Zepatier, 100 mg grazoprevir and 50 mg elbasvir, application # 208261Orig1s000) (https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208261Orig1s000ClinPharmR.pdf). 2015;3838940:1-323. Accessed 11 Jan 2018.
Geddawy A, Ibrahim YF, Elbahie NM, Ibrahim MA. Direct acting anti-hepatitis C virus drugs: clinical pharmacology and future direction. J Transl Int Med. 2017;5:8–17.
Smolders EJ, Berden FA, de Kanter CT, Kievit W, Drenth JP, Burger DM. The majority of hepatitis C patients treated with direct acting antivirals are at risk for relevant drug-drug interactions. United Eur Gastroenterol J. 2017;5:648–57.
Kiang TK, Wilby KJ, Ensom MH. Pharmacokinetic and pharmacodynamic drug interactions associated with antiretroviral drugs. 1st ed. Singapore: ADIS; 2016.
Kiang TK, Wilby KJ, Ensom MH. Clinical pharmacokinetic and pharmacodynamic drug interactions associated with antimalarials. 1st ed. Switzerland: ADIS; 2015.
Kiang TK, Wilby KJ, Ensom MH. Clinical pharmacokinetic drug interactions associated with artemisinin derivatives and HIV-antivirals. Clin Pharmacokinet. 2014;53:141–53.
Kiang TK, Wilby KJ, Ensom MH. Telaprevir: clinical pharmacokinetics, pharmacodynamics, and drug-drug interactions. Clin Pharmacokinet. 2013;52:487–510.
Kiang TK, Ensom MH, Chang TK. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol Ther. 2005;106:97–132.
Feng HP, Vaddady P, Guo Z, Liu F, Panebianco D, Levine V, Caro L, Butterton JR, Iwamoto M, Yeh WW. No pharmacokinetic interaction between the hepatitis C virus inhibitors elbasvir/grazoprevir and famotidine or pantoprazole. Clin Transl Sci. 2017;10:360–5.
Kiang TK, Ho PC, Anari MR, Tong V, Abbott FS, Chang TK. Contribution of CYP2C9, CYP2A6, and CYP2B6 to valproic acid metabolism in hepatic microsomes from individuals with the CYP2C9*1/*1 genotype. Toxicol Sci. 2006;94:261–71.
Miners JO, Mackenzie PI, Knights KM. The prediction of drug-glucuronidation parameters in humans: UDP-glucuronosyltransferase enzyme-selective substrate and inhibitor probes for reaction phenotyping and in vitro-in vivo extrapolation of drug clearance and drug-drug interaction potential. Drug Metab Rev. 2010;42:196–208.
Marshall WL, Feng HP, Wenning L, Garrett G, Huang X, Liu F, Panebianco D, Caro L, Fandozzi C, Lasseter KC, Preston RA, Marbury T, Butterton JR, Iwamoto M, Yeh WW. Pharmacokinetics, safety, and tolerability of single-dose elbasvir in participants with hepatic impairment. Eur J Drug Metab Pharmacokinet. 2017. https://doi.org/10.1007/s13318-017-0451-9 (Epub ahead of print).
Caro L, Wenning L, Guo Z, Fraser IP, Fandozzi C, Talaty J, Panebianco D, Ho M, Uemura N, Reitmann C, Angus P, Gane E, Marbury T, Smith WB, Iwamoto M, Butterton JR, Yeh WW. Effect of hepatic impairment on the pharmacokinetics of grazoprevir, a hepatitis C virus protease inhibitor. Antimicrob Agents Chemother. 2017;61:e00813–7.
United States Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research: clinical drug interaction studies—study design, data analysis, and clinical implications, guidance for industry; 2017. pp. 1–32. https://www.fda.gov/drugs/ucm292362. Accessed 11 Jan 2018.
Lau YY, Huang Y, Frassetto L, Benet LZ. Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007;81:194–204.
Annaert P, Ye ZW, Stieger B, Augustijns P. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica. 2010;40:163–76.
Kiang TK, Ensom MH. Immunosuppressants. In: Beringer PE, editor. Basic clinical pharmacokinetics. 6th ed. Wolters Kluwer; 2017, p. 320–357.
Kiang TK, Ensom MH. Anti-rejection drugs. In: Murphy JE, editor. Clinical Pharmacokinetics. 6th ed: American Society of Health-System Pharmacists; 2017. p. 205–220.
Shen H, Dai J, Liu T, Cheng Y, Chen W, Freeden C, Zhang Y, Humphreys WG, Marathe P, Lai Y. Coproporphyrins I and III as functional markers of OATP1B activity. In vitro and in vivo evaluation in preclinical species. J Pharmacol Exp Ther. 2016;357:382–93.
Marshall WL, Feng HP, Caro L, Talaty J, Guo Z, Huang X, Panebianco D, Ma J, Mangin E, O’Reilly TE, Butterton JR, Yeh WW. No clinically meaningful pharmacokinetic interaction between the hepatitis C virus inhibitors elbasvir and grazoprevir and the oral contraceptives ethinyl estradiol and levonorgestrel. Eur J Clin Pharmacol. 2017;73:593–600.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interests declared by the author.
Funding
No funding was received for the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Kiang, T.K.L. Clinical Pharmacokinetics and Drug–Drug Interactions of Elbasvir/Grazoprevir. Eur J Drug Metab Pharmacokinet 43, 509–531 (2018). https://doi.org/10.1007/s13318-018-0471-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-018-0471-0