Abstract
Background
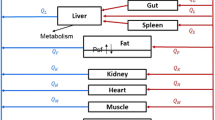
Predicting target site drug concentrations is of key importance for rank ordering compounds before proceeding to chronic pharmacodynamic models. We propose generic tumor-specific correlation-based regression equations to predict tumor-to-plasma ratios (tumor-Kps) in slow- and fast-growing xenograft mouse models.
Methods
Disposition of 14 basic small molecules was investigated extensively in mouse plasma, tissues and tumors after a single oral dose administration. Linear correlation was assessed and compared between tumor-Kp and normal tissue-to-plasma ratio (tissue-Kps) separately for each tumor xenograft. The developed regression equations were validated by leave-one-out cross-validation (LOOCV) method.
Result
Both slow- and fast-growing tumor-Kps showed good correlation (r 2 ≥ 0.7) with majority of the normal tissue-Kps. Substantial difference was observed in the slopes of developed equations between two xenografts, which was in line with observed difference in tumor distribution. The linear correlations between tumor-Kp and skin- or spleen-Kp were within the acceptable statistical criteria (LOOCV) across xenografts and the class of compounds evaluated. Since > 70% of tumor-Kps from the test data sets were predicted within a factor of twofold for both slow- and fast-growing xenograft mouse models, the results validate the applicability of the developed equations across xenografts.
Conclusion
Tumor-specific correlation-based regression equations were developed and their applicability was adequately validated across xenografts. These equations could be successfully translated to predict tumor concentrations in order to preclude experimental tumor-Kp determination.





Similar content being viewed by others
References
Abdel-Rahman SM, Kauffman RE. The integration of pharmacokinetics and pharmacodynamics: understanding dose-response. Annu Rev Pharmacol Toxicol. 2004;44:111–36.
de Lange EC, Danhof M. Considerations in the use of CSF pharmacokinetics to predict brain target concentrations in the clinical setting: implications of the barriers between blood and brain. Clin Pharmacokinet. 2002;41:691–703.
Read KD, Braggio S. Assessing brain free fraction in early drug discovery. Expert Opin Drug Metab Toxicol. 2010;6(3):337–44.
Poulin P, Theil FP. A priori prediction of tissue: plasma partition coefficients of drugs to facilitate the use of physiologically-based pharmacokinetic models in drug discovery. J Pharm Sci. 2000;89(1):16–35.
Poulin P, Theil FP. Prediction of pharmacokinetics prior to in vivo studies. 1. mechanism-based prediction of volume of distribution. J Pharm Sci. 2002;91(1):129–56.
Bj¨orkman S. Prediction of the volume of distribution of a drug: which tissue–plasma partition coefficients are needed? J Pharm Pharmacol. 2002;54(9):1237–45.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76.
Rodgers T, Leahy D, Rowland M. Tissue distribution of basic drugs: accounting for enantiomer, compound and regional difference amongst beta-blocking drugs in rat. J Pharm Sci. 2005;94(6):1237–48.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Richter WF, Starke V, Whitby B. The distribution pattern of radioactivity across different tissues in quantitative whole-body autoradiography (QWBA) studies. Eur J Pharm Sci. 2006;28(1–2):155–65.
Jansson R, Bredberg U, Ashton M. Prediction of drug tissue to plasma concentration ratios using a measured volume of distribution in combination with lipophilicity. J Pharm Sci. 2008;97(6):2324–39.
Poulin P, Theil FP. Development of a novel method for predicting human volume of distribution at steady-state of basic drugs and comparative assessment with existing methods. J Pharm Sci. 2009;98(12):4941–61.
Poulin P, Ekin S, Theil FP. A hybrid approach to advancing quantitative prediction of tissue distribution of basic drugs in human. Toxicol Appl Pharmacol. 2011;250(2):194–212.
Graham H, Walker M, Jones O, Yates J, Galetin A, Aarons L. Comparison of in vivo and in silico methods used for prediction of tissue: plasma partition coefficients in rat. J Pharm Pharmacol. 2012;64(3):383–96.
Poulin P, Dambach DM, Hartley DH, Ford K, Theil FP, Harstad E, Halladay J, Choo E, Boggs J, Liederer BM, Dean B, Diaz D. An algorithm for evaluating potential tissue drug distribution in toxicology studies from readily available pharmacokinetic parameters. J Phar Sci. 2013;102(10):3816–29.
Yun YE, Edginton AN. Correlation-based prediction of tissue-to plasma partition coefficients using readily available input parameters. Xenobiotica. 2013;43(10):839–52.
Poulin P, Hop CE, Salphati L, Liederer BM. Correlation of tissue-to-plasma partition coefficient between normal tissues and subcutaneous xenografts of human tumor cell lines in mouse as a prediction tool of drug penetration in tumors. J Pharm Sci. 2013;102(4):1355–69.
Patrick P, Chen YH, Ding X, Gould SE, Hop CE, Messick K, Oeh J, Liederer BM. Prediction of drug distribution in subcutaneous xenografts of human tumor cell lines and healthy tissues in mouse: application of the tissue composition-based model to antineoplastic drugs. J Pharm Sci. 2015;104(4):1508–21.
Williamson MJ, Silva MD, Terkelsen J, Robertson R, Yu L, Xia C, Hatsis P, Bannerman B, Babcock T, Cao Y, Kupperman E. The relationship among tumor architecture, pharmacokinetics, pharmacodynamics, and efficacy of bortezomib in mouse xenograft models. Mol Cancer Ther. 2009;8(12):3234–43.
Nigade PB, Gundu J, Pai KS, Nemmani KV. Prediction of tissue-to-plasma ratios of basic compounds in mice. Eur J Drug Metab Pharmacokinet. 2017;42(5):835–47.
Moore DS, Notz WI, Flinger MA. The basic practice of statistics. 6th ed. New York: W. H. Freeman and Company; 2013. p. 138.
Kiralj R, Ferreira MC. Basic validation procedures models in QSAR and QSPR studies: theory and application. J Braz Chem Soc. 2009;20(4):770–87.
The Report From the Expert Group on (Quantitative) Structure-Activity Relationships [(Q)Sars] on the Principles for the Validation Of (Q)Sars. OECD Environment Health and Safety Publications Series on Testing and Assessment No. 49. OECD: Paris, 2004.
Guidance Document on the Validation of (Quantitative) Structure-Activity Relationship [(Q)SAR] Models. OECD Environment Health and Safety Publications Series on Testing and Assessment No. 69. OECD: Paris, 2007.
Downey CM, Singla AK, Villemaire ML, Buie HR, Boyd SK, Jirik FR. Quantitative ex-vivo micro-computed tomographic imaging of blood vessels and necrotic regions within tumors. PLoS One. 2012. https://doi.org/10.1371/journal.pone.0041685.
Kallinowski F, Schlenger KH, Runkel S, Kloes M, Stohrer M, Okunieff P, Vaupel P. Blood flow, metabolism, cellular microenvironment, and growth rate of human tumor xenografts. Cancer Res. 1989;49(14):3759–64.
Saleem A, Price PM. Early tumor drug pharmacokinetics is influenced by tumor perfusion but not plasma drug exposure. Clin Cancer Res. 2008;14(24):8184–90.
Raghunand N, Mahoney BP, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. II. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem Pharmacol. 2003;66(7):1219–29.
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–84.
Kazmi F, Hensley T, Pope C, Funk RS, Loewen GJ, Buckley DB, Parkinson A. Lysosomal sequestration (trapping) of lipophilic amine (cationic amphiphilic) drugs in immortalized human hepatocytes (Fa2N-4 cells). Drug Metab Dispos. 2013;41(4):897–905.
Bradshaw-Pierce EL, Pitts TM, Tan AC, McPhillips K, West M, Gustafson DL, Halsey C, Nguyen L, Lee NV, Kan JL, Murray BW, Eckhardt SG. Tumor p-glycoprotein correlates with efficacy of PF-3758309 in in vitro and in vivo models of colorectal cancer. Front Pharmacol. 2013;4:22. https://doi.org/10.3389/fphar.2013.00022.
Heffron TP. Small molecule kinase inhibitors for the treatment of brain cancer. J Med Chem. 2016;59(22):10030–66.
Acknowledgements
We are grateful for the support of senior management of Lupin Limited (Research Park), Pune, India, and technical assistance of Mr. Tariq Bhat and Dr. Praveen Kumar during development of human xenograft mouse models.
Funding
The present work was supported by and conducted at Lupin Ltd (Research Park), Pune.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Prashant B. Nigade, Jayasagar Gundu, K. Sreedhara Pai and Kumar V.S. Nemmani have no conflict of interest.
Ethical approval
In vivo studies were performed at the AAALAC accredited facility (Lupin Limited, Pune, India) in accordance with the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) guidelines and as per Institutional Animal Ethics Committee (IAEC) approved experimental protocols numbers: IAEC/PK/534, IAEC/PK/619 and IAEC/PK/620.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nigade, P.B., Gundu, J., Pai, K.S. et al. Prediction of Tumor-to-Plasma Ratios of Basic Compounds in Subcutaneous Xenograft Mouse Models. Eur J Drug Metab Pharmacokinet 43, 331–346 (2018). https://doi.org/10.1007/s13318-017-0454-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-017-0454-6