Abstract
Background and Objective
For patients with intracranial infection, local intrathecal administration of meropenem may be a useful method to obtain a sufficient drug concentration in the cerebral spinal fluid (CSF). However, a large inter-individual variability may pose treatment efficacy at risk. This study aimed to identify factors affecting drug concentration in the CSF using population pharmacokinetics method.
Methods
After craniotomy, aneurysm patients with an indwelling lumbar cistern drainage tube who received a combined intravenous and intrathecal administration of meropenem for the treatment of suspected intracranial infection were enrolled. Venous blood and CSF specimens were collected for determining meropenem concentrations. Nonlinear mixed-effects modeling method was used to fit blood and CSF concentrations simultaneously and to develop the population pharmacokinetic model. The proposed model was applied to simulate dosage regimens.
Results
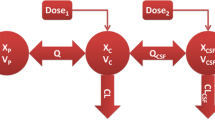
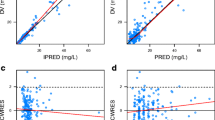
A three-compartmental model was established to describe meropenem in vivo behavior. Lumbar CSF drainage resulted in a drug loss, and drug clearance in CSF (CLCSF) was employed to describe this. The covariate analysis found that the drainage volume (mL/day) was strongly associated with CLCSF, and the effect of creatinine clearance was significant on the clearance of meropenem in blood (CL). Visual predictive check suggested that the proposed pharmacokinetic model agreed well with the observations. Simulation showed that both intravenous and intrathecal doses should be increased with the increases of minimum inhibitory concentration and daily CSF drainage volume.
Conclusion
This model incorporates covariates of the creatinine clearance and the drainage volume, and a simple to use dosage regimen table was created to guide clinicians with meropenem dosing.



Similar content being viewed by others
References
National Nosocomial Infections Surveillance S. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85.
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267–84.
Beer R, Lackner P, Pfausler B, Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol. 2008;255:1617–24.
Rifi L, Barkat A, El Khamlichi A, Boulaadas M, El Ouahabi A. Neurosurgical management of anterior meningo-encephaloceles about 60 cases. Pan Afr Med J. 2015;21:215.
Zolal A, Juratli T, Dengl M, Ficici KH, Schackert G, Sobottka SB. Daily drained CSF volume is a predictor for shunt dependence—a retrospective study. Clin Neurol Neurosurg. 2015;138:147–50.
Al-Tamimi YZ, Bhargava D, Feltbower RG, Hall G, Goddard AJ, Quinn AC, et al. Lumbar drainage of cerebrospinal fluid after aneurysmal subarachnoid hemorrhage: a prospective, randomized, controlled trial (LUMAS). Stroke. 2012;43:677–82.
Park S, Yang N, Seo E. The effectiveness of lumbar cerebrospinal fluid drainage to reduce the cerebral vasospasm after surgical clipping for aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc. 2015;57:167–73.
Li X, Wu Y, Sun S, Mei S, Wang J, Wang Q, et al. Population pharmacokinetics of vancomycin in postoperative neurosurgical patients. J Pharm Sci. 2015;104:3960–7.
Chen K, Wu Y, Wang Q, Wang J, Li X, Zhao Z, et al. The methodology and pharmacokinetics study of intraventricular administration of vancomycin in patients with intracranial infections after craniotomy. J Crit Care. 2015;30(218):e1–5.
Gutierrez J, Federici T, Peterson B, Bartus R, Betourne A, Boulis NM. Development of intrathecal riluzole: a new route of administration for the treatment of amyotrophic lateral sclerosis patients. Neurosurgery. 2016;63(Suppl 1):193.
Mei S, Luo X, Li X, Li Q, Huo J, Yang L, et al. Development and validation of an LC-MS/MS method for the determination of tigecycline in human plasma and cerebrospinal fluid and its application to a pharmacokinetic study. Biomed Chromatogr. 2016;30:1992–2002.
Blassmann U, Roehr AC, Frey OR, Vetter-Kerkhoff C, Thon N, Hope W, et al. Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: a prospective observational study. Crit Care. 2016;20:343.
Zhang Y, Zhang J, Chen Y, Yu J, Cao G, Wu X, et al. Evaluation of meropenem penetration into cerebrospinal fluid in patients with meningitis after neurosurgery. World Neurosurg. 2017;98:525–31.
Roth T, Fiedler S, Mihai S, Parsch H. Determination of meropenem levels in human serum by high-performance liquid chromatography with ultraviolet detection. Biomed Chromatogr. 2017. doi:10.1002/bmc.3880.
Li X, Wu Y, Sun S, Zhao Z, Wang Q. Population pharmacokinetics of vancomycin in postoperative neurosurgical patients and the application in dosing recommendation. J Pharm Sci. 2016;105:3425–31.
Lu C, Zhang Y, Chen M, Zhong P, Chen Y, Yu J, et al. Population pharmacokinetics and dosing regimen optimization of meropenem in cerebrospinal fluid and plasma in patients with meningitis after neurosurgery. Antimicrob Agents Chemother. 2016;60:6619–25.
Lodise TP, Nau R, Kinzig M, Drusano GL, Jones RN, Sorgel F. Pharmacodynamics of ceftazidime and meropenem in cerebrospinal fluid: results of population pharmacokinetic modelling and Monte Carlo simulation. J Antimicrob Chemother. 2007;60:1038–44.
Li C, Kuti JL, Nightingale CH, Nicolau DP. Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J Clin Pharmacol. 2006;46:1171–8.
Chung EK, Cheatham SC, Fleming MR, Healy DP, Kays MB. Population pharmacokinetics and pharmacodynamics of meropenem in nonobese, obese, and morbidly obese patients. J Clin Pharmacol. 2017;57:356–68.
Usman M, Frey OR, Hempel G. Population pharmacokinetics of meropenem in elderly patients: dosing simulations based on renal function. Eur J Clin Pharmacol. 2017;73:333–42.
Xu Q, Yu SB, Zheng N, Yuan XY, Chi YY, Liu C, et al. Head movement, an important contributor to human cerebrospinal fluid circulation. Sci Rep. 2016;6:31787.
Acknowledgements
We would like to thank Dr. Yuanxing Wu and Dr. Guangqiang Chen for their help with sample collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Grants from the National Natural Science Foundation of China (81503157), the Organization Department of Beijing Municipal Committee (2014000021469G258), and the Capital Medical University (16JL72).
Conflicts of interest
XL, SS, QW and ZZ have no conflict of interest to declare.
Ethics approval
All procedures in this study were in accordance with the 2008 Declaration of Helsinki and ICH Guideline for Good Clinical Practice. This protocol was approved by the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University (ID: KY2014-014-02).
Informed consent
Written informed consents from all patients or their healthcare surrogates were obtained.
Rights and permissions
About this article
Cite this article
Li, X., Sun, S., Wang, Q. et al. Population Pharmacokinetics of Combined Intravenous and Local Intrathecal Administration of Meropenem in Aneurysm Patients with Suspected Intracranial Infections After Craniotomy. Eur J Drug Metab Pharmacokinet 43, 45–53 (2018). https://doi.org/10.1007/s13318-017-0422-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-017-0422-1