Abstract
Background and Objectives
Clopidogrel is an antiplatelet and antithrombotic prodrug. It has poor oral bioavailability due to poor dissolution and possible premature degradation in the intestine. Accordingly, the objective of this study was to enhance clopidogrel dissolution rate and to reduce its premature degradation in rabbit intestine.
Methods
Solid dispersion (SD) systems of clopidogrel with gelucire 50/13 and/or cremophor RH40 were prepared using fusion technique. The SD systems were characterized with respect to drug dissolution. The characterization included thermal analysis and infrared investigations. The stability of clopidogrel in the fluid extracted from small intestinal and colonic mucosal surfaces was monitored both in absence and presence of cremophor or gelucire.
Results
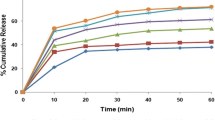
SD formation enhanced drug dissolution with the enhancement increasing at higher concentrations of either cremophor or gelucire. The ternary SD of clopidogrel with cremophor and gelucire reflected synergism between them. This synergism was manifested by enhanced dissolution efficiency of drug to reach 85 % at pH 6.8 and 89 % at pH 7.4 compared to unprocessed drug which liberated 16.2 and 15.2 % at the same pH values, respectively. Enhanced dissolution from SD was mainly due to micellar solubilization for cremophor and was due to change in the crystalline nature of drug with a contribution to self-emulsification in case of gelucire. Clopidogrel showed premature degradation in the intestinal fluid. Cremophor RH 40 reduced this degradation but gelucire failed in this respect.
Conclusion
The study introduced SD system for enhanced dissolution rate of clopidogrel with a potential of reduced premature degradation in the intestine.









Similar content being viewed by others
References
Lassoued MA, Sfar S, Bouraoui A, Khemiss F. Absorption enhancement studies of clopidogrel hydrogen sulphate in rat everted gut sacs. J Pharm Pharmacol. 2012;64:541–52.
Fox KA, Chelliah R. Clopidogrel: an updated and comprehensive review. Expert Opin Drug Metab Toxicol. 2007;3:621–31.
European Medicines agency Evaluation of medicines for human use. Doc. Ref EMEA/661461/2009.
Zupancic V, Smrkolj M, Benkic P, Simonic I, Plevnik M, Ritlop G, Kristl A, Vrecer F. Preformulation investigation of some clopidogrel addition salts. Acta Chim Slov. 2010;57:376–85.
Singh SK, Som S, Shankhwar U. Formulation and optimization of solid dispersion of Clopidogrel with PEG 6000. J Appl Pharm Sci. 2011;01(08):217–26.
Bouman HJ, Schömig E, Werkum JWV, Velder J, Hackeng CM, Hirschhäuser C, Waldmann C, Schmalz GH, Berg JMT, Taubert D. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med. 2011;17(1):110–6.
Stoeckel K, Hofheinz W, Laneury JP, Duchene P, Shedlofsky S, Blouin RA. Stability of cephalosporin prodrug esters in human intestinal juice: implications for oral bioavailability. Antimicrob Agents Chemother. 1998;42:2602–6.
Masaki K, Hashimoto M, Imai T. Intestinal first-pass metabolism via carboxylesterase in rat jejunum and ileum. J Pharmacol Exp Ther. 2007;35:1089–95.
Borde AS, Karlsson EM, Andersson K, Bjorhall K, Lennernas H, Abrahamsson B. Assessment of enzymatic prodrug stability in human, dog and simulated intestinal fluids. Eur J Pharm Biopharm. 2012;80:630–7.
Ohura K, Soejima T, Nogata R, Adachi Y, Ninomiya S, ImAai T. Effect of intestinal first-pass hydrolysis on the oral bioavailability of an ester prodrug of fexofenadine. J Pharm Sci. 2012;101(9):3264–74.
Validation of analytical procedures: methodology. ICH Harmonized Tripartite Guidline. 1996.
Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27:48–9.
Koradia V, Chawla G, Bansal AK. Qualitative and quantitative analysis of clopidogrel bisulphate polymorphs. Acta Pharm. 2004;54:193–204.
Eloy JDO, Saraiva J, Albuquerque SD, Marchetti JM. Solid dispersion of ursolic acid in gelucire 50/13: a strategy to enhance drug release and trypanocidal activity. AAPS PharmSciTech. 2012;13(4):1436–45.
El-Gizawy SA, Osman MA, Arafa MF, El Maghraby GM. Aerosil as a novel co-crystal co-former for improving the dissolution rate of hydrochlorothiazide. Int J Pharm. 2015;478:773–8.
Azubuike CP, Okhamafe AO. Physicochemical, spectroscopic and thermal properties of microcrystalline cellulose derived from corn cobs. Int J Recycl Org Waste Agric. 2012;1(9):1–7.
El-Badry M. Physicochemical characterization and dissolution properties of meloxicam-gelucire 50/13 binary systems. Sci Pharm. 2011;79:375–86.
Ibrahim MA, El-Badry M. Formulation of immediate release pellets containing famotidine solid dispersions. Saudi Pharm J. 2014;22:149–56.
Rashmika B, Veena V, Kachhwaha S, Bhikshapathi DVRN. Formulation development and in vivo evaluation of fexofenadine HCl solid dispersions by spray drying technique. Der Pharm Lett. 2013;5(6):73–82.
Maheswari PD, Rambhau D, Narasu ML. Development of valsartan controlled release formulations from spray dried powder by micellar solubilization technique. Inter J Adv Pharm Res. 2014;5(3):157–68.
Rus LM, Tomuta I, Iuga C, Maier C, Kacso I, Borodi G, Bratu I, Bojita M. Compatability studies of indapamide/pharmaceutical excipients used in tablet preformulation. Farmacia. 2012;60:92–101.
Pachuau L, Malsawmtluangi C, Nath NK, Ramdinsangi H, Vanlalfakawma DC, Tripathi SK. Physicochemical and functional characterization of microcrystalline cellulose from bamboo (Dendrocalamus longispathus). Inter J Pharm Tech Res. 2013;5(4):1561–71.
Pimple S, Maurya P, Joshi A, Jain A, Gurjar M, Shah M. Formulation and in vitro evaluation of immediate release tablets containing antiplatelet drug: clopidogrel. World J Pharm Pharm Sci. 2014;3(8):2007–19.
EL Maghraby GM, Alomrani AH. Synergistic enhancement of itraconazole dissolution by ternary system formation with pluronic F68 and hydroxypropylmethylcellulose. Sci Pharm. 2009;77:401–17.
Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21(2):201–30.
Kim DW, Kwon MS, Yousaf AM, Balakrishnan P, Park JH, Kim DS, Lee BJ, Park YJ, Yong CS, Kim JO, Choia HG. Comparison of a solid SMEDDS and solid dispersion for enhanced stability and bioavailability of clopidogrel napadisilate. Carbohydr Polym. 2014;114:365–74.
Patel P, Mehta T, Panchal S. Preparation, evaluation and comparison of lipid based drug delivery systems of tacrolimus. Int J Pharm Pharm Sci. 2014;6:588–91.
Patel N, Dalrymple DM, Serajuddin ATM. Development of solid SEDDS, III: application of Acconon® C-50 and Gelucire® 50/13 as both solidifying and emulsifying agents for medium chain triglycerides. J Excip Food Chem. 2012;3(2):83–92.
Schiel MA, Green SL, Davis WI, Sanghani PC, Bosron WF, Sanghani SP. Expression and characterization of a human carboxylesterase 2 splice variant. J Pharmacol Exp Ther. 2007;323:94–101.
Taketani M, Shii M, Ohura K, Ninomiya S, Imai T. Carboxylesterase in the liver and small intestine of experimental animals and human. Life Sci. 2007;81:924–32.
Zhang C, Xu Y, Zhong Q, Li X, Gao P, Feng C, Chu Q, Chen Y, Liu D. In vitro evaluation of the inhibitory potential of pharmaceutical excipients on human carboxylesterase 1A and 2. Plos one. 2014;9:1–8.
Granero GE, Amidon GL. Stability of valacyclovir: implications for its oral bioavailability. Int J Pharm. 2006;317:14–8.
Werdenberga D, Joshib R, Wolfframc S, Merklea HP, Langguthd P. Presystemic metabolism and intestinal absorption of antipsoriatic fumaric acid esters. Biopharm Drug Dispos. 2003;24:259–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Source of funding
The work is a part of the Master thesis of Dina Bali and utilized the resources of College of Pharmacy, Tanta University.
Conflict of interest
DEB, MAO and GMEM have no conflicts of interest.
Ethical approval
The study protocol was approved by the College of Pharmacy Ethical Committee.
Rights and permissions
About this article
Cite this article
Bali, D.E., Osman, M.A. & El Maghraby, G.M. Enhancement of Dissolution Rate and Intestinal Stability of Clopidogrel Hydrogen Sulfate. Eur J Drug Metab Pharmacokinet 41, 807–818 (2016). https://doi.org/10.1007/s13318-015-0311-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-015-0311-4