Abstract
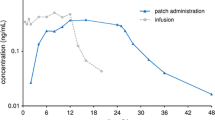
The transdermally applied dopamine receptor agonist rotigotine is extensively metabolized in the liver. An open-label, parallel-group study was conducted to evaluate the effects of moderate hepatic impairment on the pharmacokinetics, safety and tolerability of rotigotine. Eight subjects with normal hepatic function and nine with moderate hepatic impairment (Child–Pugh class B) received one rotigotine transdermal patch (providing a dose of 2 mg/24 h) daily for 3 days with a 24-h patch-on period. Blood and urine samples were collected to evaluate pharmacokinetic parameters characterizing drug bioavailability and elimination. Primary variables included plasma and urine concentrations of unconjugated rotigotine (active parent compound) and total rotigotine (unconjugated rotigotine plus sulfate and glucuronide conjugates) under steady-state (SS) conditions. For unconjugated rotigotine, point estimates for the ratios of AUC(0–24)SS and C max,SS between the two groups (normal vs. impaired hepatic function) were near 1: AUC(0–24)SS, 0.90 (90 % CI 0.59, 1.38) and C max,SS, 0.94 (90 % CI 0.66, 1.35); t max,SS and t 1/2 were lower in subjects with hepatic impairment, while renal clearance was unaffected and overall clearance was higher. For total rotigotine, C max,SS was higher in subjects with hepatic impairment compared with those with normal hepatic function (P = 0.0239, ANOVA). A tendency to reduced non-renal clearance was observed in subjects with hepatic impairment, consistent with their higher plasma concentrations of total rotigotine. Thus, moderate hepatic impairment did not influence the pharmacokinetics of unconjugated rotigotine under steady-state conditions suggesting that dose adjustment will not be required for patients with mild or moderate hepatic insufficiency. In addition, the rotigotine patch was well tolerated in subjects with moderate hepatic impairment.


Similar content being viewed by others
References
Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J et al (2003) Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 4:101–119
Bergquist C, Lindegård J, Salmonson T (1999) Dosing recommendations in liver disease. Clin Pharmacol Ther 66:201–204
Boyd RA, Yang BB, Abel RB, Eldon MA, Sedman AJ, Forgue ST (1996) Pharmacokinetics of a 7-day 17β-estradiol transdermal delivery system: effect of application site and repeated applications on serum concentrations of estradiol and estrone. J Clin Pharmacol 36:998–1005
Braun M, Cawello W, Boekens H, Horstmann R (2009a) Influence of domperidone on pharmacokinetics, safety and tolerability of the dopamine agonist rotigotine. Br J Clin Pharmacol 67:209–215
Braun M, Cawello W, Andreas JO, Boekens H, Horstmann R (2009b) Lack of pharmacokinetic interactions between transdermal rotigotine and oral levodopa/carbidopa. J Clin Pharmacol 49:1047–1055
Cawello W, Wolff HM, Meuling WJ, Horstmann R, Braun M (2007) Transdermal administration of radiolabelled [14C]rotigotine by a patch formulation: a mass balance trial. Clin Pharmacokinet 46:851–857
Cawello W, Braun M, Boekens H (2009) Absorption, disposition, metabolic fate, and elimination of the dopamine agonist rotigotine in man: administration by intravenous infusion or transdermal delivery. Drug Metab Dispos 37:2055–2060
Cawello W, Ahrweiler S, Sulowicz W, Szymczakiewicz-Multanowska A, Braun M (2012) Single-dose pharmacokinetics of the transdermal rotigotine patch in patients with impaired renal function. Br J Clin Pharmacol 73:46–54
Chase TN (1998) Levodopa therapy: consequences of the nonphysiologic replacement of dopamine. Neurology 50:S17–S25
European Medicines Agency (2005) Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with impaired hepatic function. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003122.pdf. Accessed 12 March 2013
Garcia-Borreguero D, Stillman P, Benes H, Buschmann H, Chaudhuri KR, Gonzalez Rodriguez VM, Hogl B, Kohnen R, Monti GC, Stiasny-Kolster K, Trenkwalder C, Williams AM, Zucconi M (2011) Algorithms for the diagnosis and treatment of restless legs syndrome in primary care. BMC Neurol 11:28
Hening WA, Allen RP, Ondo WG, Walters AS, Winkelman JW, Becker P, Bogan R, Fry JM, Kudrow DB, Lesh KW, Fichtner A, Schollmayer E, On behalf of the SP792 Study Group (2010) Rotigotine improves restless legs syndrome: a 6-month randomized double-blind placebo-controlled trial in the United States. Mov Disord 25:1675–1683
Jenner P (2005) A novel dopamine agonist for the transdermal treatment of Parkinson’s disease. Neurology 65:S3–S5
LeWitt PA, Lyons KE, Pahwa R (2007) Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology 68:1262–1267
McLean AJ, Le Couteur DG (2004) Aging biology and geriatric clinical pharmacology. Pharmacol Rev 56:163–184
Obeso JA, Rodriguez-Oroz M, Marin C, Alonso F, Zamarbide I, Lanciego JL, Rodriguez-Diaz M (2004) The origin of motor fluctuations in Parkinson’s disease: importance of dopaminergic innervation and basal ganglia circuits. Neurology 62:S17–S30
Oertel W, Trenkwalder C, Benes H, Ferini-Strambi L, Hogl B, Poewe W, Stiasny-Kolster K, Fichtner A, Schollmayer E, Kohnen R, Garcia-Borreguero D (2011) Long-term safety and efficacy of rotigotine transdermal patch for moderate-to-severe idiopathic restless legs syndrome: a 5-year open-label extension study. Lancet Neurol 10:710–720
Parkinson Study Group (2003) A controlled trial of rotigotine monotherapy in early Parkinson’s disease. Arch Neurol 60:1721–1728
Perez-Lloret S, Rascol O (2010) Dopamine receptor agonists for the treatment of early or advanced Parkinson’s disease. CNS Drugs 24:941–968
Poewe WH, Rascol O, Quinn N, Tolosa E, Oertel WH, Martignoni E, Rupp M, Boroojerdi B (2007) Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson’s disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol 6:513–520
Rascol O, Perez-Lloret S (2009) Rotigotine transdermal delivery for the treatment of Parkinson’s disease. Expert Opin Pharmacother 10:677–691
Rodighiero V (1999) Effects of liver disease on pharmacokinetics. An update. Clin Pharmacokinet 37:399–431
Scheller D, Dürmüller N, Moser P, Porsolt RD (2008) Continuous stimulation of dopaminergic receptors by rotigotine does not interfere with the sleep–wake cycle in the rat. Eur J Pharmacol 584:111–117
Schmidt WJ, Lebsanft H, Heindl M, Gerlach M, Gruenblatt E, Riederer P, Mayerhofer A, Scheller DK (2008) Continuous versus pulsatile administration of rotigotine in 6-OHDA-lesioned rats: contralateral rotations and abnormal involuntary movements. J Neural Transm 115:1385–1392
Scholz H, Trenkwalder C, Kohnen R, Riemann D, Kriston L, Hornyak M (2011) Dopamine agonists for restless legs syndrome. Cochrane Database Syst Rev 16(3):CD006009. doi:10.1002/14651858.CD006009.pub2
Solassol I, Bressolle F, Caumette L, Garcia F, Poujol S, Culine S, Pinguet F (2005) Inter- and intraindividual variabilities in pharmacokinetics of fentanyl after repeated 72-hour transdermal applications in cancer pain patients. Ther Drug Monit 27:491–498
Swart PJ, Bronner GM, Bruins AP, Ensing K, Tepper PG, De Zeeuw RA (1993) HPLC-UV atmospheric pressure ionization mass spectrometry determination of the dopamine D2 agonist N-0923 and its major metabolites after oxidative metabolism by rat-, monkey-, and human liver microsomes. Toxicol Mech Methods 3:279–290
Swart PJ, Oelen WE, Bruins AP, Tepper PG, de Zeeuw RA (1994) Determination of the dopamine D2 agonist N-0923 and its major metabolites in perfused rat livers by HPLC-UV-atmospheric pressure ionization mass spectrometry. J Anal Toxicol 18:71–77
Thummel KE, Shen DD (2006) Design and optimization of dosage regimes: pharmacokinetic data. In: Brunton LL, Lazo JS, Parker KL (eds) Goodman and Gilmans’ the pharmacological basis of therapeutics, 11th edn. McGraw-Hill, New York, pp 1917–2023
Trenkwalder C, Hening WA, Montagna P, Oertel WH, Allen RP, Walters AS, Costa J, Stiasny-Kolster K, Sampaio C (2008a) Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord 23:2267–2302
Trenkwalder C, Benes H, Poewe W, Oertel WH, Garcia-Borreguero D, de Weerd AW, Ferini-Strambi L, Montagna P, Odin P, Stiasny-Kolster K, Hogl B, Chaudhuri KR, Partinen M, Schollmayer E, Kohnen R (2008b) Efficacy of rotigotine for treatment of moderate-to-severe restless legs syndrome: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 7:595–604
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) (1998) Guidance for industry. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072123.pdf. Accessed 12 March 2013
Watts RL, Wendt J, Nausieda PL, Boroojerdi B, Poole KH, Sommerville KW (2004) Efficacy, safety, and tolerability of the rotigotine transdermal patch in patients with early-stage, idiopathic Parkinson’s disease: a multicenter, multinational, randomized, double-blind, placebo-controlled trial. Mov Disord 19:S258
Acknowledgments
W.C., A.F., H.B. and M.B. are employees of the study sponsor, UCB Pharma, Monheim am Rhein, Germany. The authors acknowledge the participation of Faculty Derer’s Hospital, Bratislava, Slovak Republic who conducted the trial. AAI Deutschland GmbH & Co. KG (now NUVISAN GmbH), Neu-Ulm, Germany performed all bioanalytical work to quantify rotigotine and metabolites in plasma and urine samples. In addition, AAI determined protein binding in selected plasma samples. Data management and statistical analyses were performed by Independent Clinical Research Consulting, Berlin, Germany and study conduct was coordinated by Pharmacon Research GmbH, Berlin, Germany. Writing and editorial support was provided by Birgit Brett (Brett Medical Writing, Pulheim, Germany) and Aideen Young, Ph.D. (Evidence Scientific Solutions, London, UK) and contracted by UCB Pharma, Brussels, Belgium. The authors also acknowledge Ging-Ging Li, CMPP (Global Publications Manager, Movement & Sleep Disorders, UCB Pharma, Brussels, Belgium) for publication coordination. All costs associated with the development and the publishing of the present manuscript were met by the sponsor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cawello, W., Fichtner, A., Boekens, H. et al. Influence of hepatic impairment on the pharmacokinetics of the dopamine agonist rotigotine. Eur J Drug Metab Pharmacokinet 39, 155–163 (2014). https://doi.org/10.1007/s13318-013-0153-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-013-0153-x