Abstract
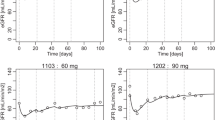
Magnesium isoglycyrrhizinate (MI) has been complementarily used for restoring the hepatic impairments caused by taxol plus platinum based chemotherapies in China. Due to the hepatic dependence of paclitaxel elimination, this pilot clinical study aimed to investigate the influence of MI on the pharmacokinetics of paclitaxel in epithelial ovarian cancer patients. During the standard chemotherapy of intravenous paclitaxel (125 mg/m2 infused over a 3-h period) and intraperitoneal cisplatin (60 mg/m2) for patients with FIGO stage II epithelial ovarian cancer, 9 each of total 18 patients were respectively treated with intravenous MI (100 mg) or vehicle control for 4 days. Plasma paclitaxel was analyzed by HPLC and the pharmacokinetic parameters were calculated with non-compartmental analysis. The hematological, hepatic and renal status was monitored before and 3 days after paclitaxel administration. It was observed the terminal t 1/2 and MRT of paclitaxel were significantly (p = 0.002 and 0.001) reduced by MI, respectively, from 11.0 ± 2.2 and 5.6 ± 1.0 h to 7.7 ± 1.7 and 4.0 ± 0.3 h. Hematological toxicity indicated by platelet count and hepatic events marked with ALT, AST and γ-GT were significant in both groups. In spite of the insignificance of decreased system exposure of paclitaxel and recovered hepatic function by MI, they did correlate with each other. It was therefore deduced that the liver toxicities of paclitaxel plus cisplatin chemotherapy potentially decrease hepatic elimination and increase system exposure of paclitaxel, and the recovery of liver function by MI helps to restore hepatic clearance of paclitaxel. The clinical significance of this pharmacokinetic interaction requires further studies with larger population size.

Similar content being viewed by others
References
Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H (1997) The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 79(8):1494–1500
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354(1):34–43
Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F (2001) Assessment of neurotoxicity following repeated cremophor/ethanol injections in rats. Neurotox Res 3(3):301–306
Briasoulis E, Karavasilis V, Tzamakou E, Piperidou C, Soulti K, Pavlidis N (2006) Feasibility study and pharmacokinetics of low-dose paclitaxel in cancer patients with severe hepatic dysfunction. Anticancer Drugs 17(10):1219–1222
Bristow RE, Santillan A, Salani R, Diaz-Montes TP, Giuntoli RL II, Meisner BC, Armstrong DK, Frick KD (2007) Intraperitoneal cisplatin and paclitaxel versus intravenous carboplatin and paclitaxel chemotherapy for stage III ovarian cancer: a cost-effectiveness analysis. Gynecol Oncol 106(3):476–481
Cheng Y, Zhang J, Shang J, Zhang L (2009) Prevention of free fatty acid-induced hepatic lipotoxicity in HepG2 cells by magnesium isoglycyrrhizinate in vitro. Pharmacology 84(3):183–190
de Jongh FE, de Wit R, Verweij J, Sparreboom A, van den Bent MJ, Stoter G, van der Burg ME (2002) Dose-dense cisplatin/paclitaxel. a well-tolerated and highly effective chemotherapeutic regimen in patients with advanced ovarian cancer. Eur J Cancer 38(15):2005–2013
du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, Bauknecht T, Richter B, Warm M, Schroder W, Olbricht S, Nitz U, Jackisch C, Emons G, Wagner U, Kuhn W, Pfisterer J (2003) A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst 95(17):1320–1329
Harris JW, Rahman A, Kim BR, Guengerich FP, Collins JM (1994) Metabolism of taxol by human hepatic microsomes and liver slices: participation of cytochrome P450 3A4 and an unknown P450 enzyme. Cancer Res 54(15):4026–4035
Huizing MT, Sparreboom A, Rosing H, van Tellingen O, Pinedo HM, Beijnen JH (1995) Quantification of paclitaxel metabolites in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Appl 674(2):261–268
Jiko M, Yano I, Okuda M, Inui K (2005) Altered pharmacokinetics of paclitaxel in experimental hepatic or renal failure. Pharm Res 22(2):228–234
Kiso Y, Tohkin M, Hikino H, Hattori M, Sakamoto T, Namba T (1984) Mechanism of antihepatotoxic activity of glycyrrhizin. I: effect on free radical generation and lipid peroxidation. Planta Med 50(4):298–302
Kurata T, Tamura T, Shinkai T, Ohe Y, Kunitoh H, Kodama T, Kakinuma R, Matsumoto T, Kubota K, Omatsu H, Nishiwaki Y, Saijo N (2001) Phase I and pharmacological study of paclitaxel given over 3 h with cisplatin for advanced non-small cell lung cancer. Jpn J Clin Oncol 31(3):93–99
Lagas JS, Vlaming ML, van Tellingen O, Wagenaar E, Jansen RS, Rosing H, Beijnen JH, Schinkel AH (2006) Multidrug resistance protein 2 is an important determinant of paclitaxel pharmacokinetics. Clin Cancer Res 12(20 Pt 1):6125–6132
Landrum LM, Hyde J Jr, Mannel RS, McMeekin DS, Moore KN, Walker JL (2011) Phase II trial of intraperitoneal cisplatin combined with intravenous paclitaxel in patients with ovarian, primary peritoneal and fallopian tube cancer. Gynecol Oncol 122(3):527–531
Liebmann J, Cook JA, Mitchell JB (1993) Cremophor EL, solvent for paclitaxel, and toxicity. Lancet 342(8884):1428
Makhija S, Sabbatini P, Aghajanian C, Venkatraman E, Spriggs DR, Barakat R (2000) Intraperitoneal cisplatin and intravenous paclitaxel in the treatment of epithelial ovarian cancer patients with a positive second look. Gynecol Oncol 79(1):28–32
Malingre MM, Beijnen JH, Rosing H, Koopman FJ, Jewell RC, Paul EM, Ten Bokkel Huinink WW, Schellens JH (2001) Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br J Cancer 84(1):42–47
Mao YM, Lu LG, Cai X, Wu SM, Chen CW, Fu QC, Cao AP, Chen HZ, Zeng MD (2009a) Magnesium isoglycyrrhizinate in the treatment of chronic liver diseases with high ALT level: a randomized, double-blind, placebo controlled, mult-i center, dose-finding study. Chin Hepatol 14(6):442–445
Mao YM, Zeng MD, Chen Y, Chen CW, Fu QC, Cai X, Wu SM, Chen YG, Sun Y, Li J, Sui YH, Zhao W, Lu LG, Cao AP, Chen HZ (2009b) Magnesium isoglycyrrhizinate in the treatment of chronic liver diseases: a randomized, double-blind, multi-doses, active drug controlled, multi-center study. Zhonghua Gan Zang Bing Za Zhi 17(11):847–851
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M (1996) Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 334(1):1–6
Meerum Terwogt JM, Beijnen JH, ten Bokkel Huinink WW, Rosing H, Schellens JH (1998) Co-administration of cyclosporin enables oral therapy with paclitaxel. Lancet 352(9124):285
Monsarrat B, Alvinerie P, Wright M, Dubois J, Gueritte-Voegelein F, Guenard D, Donehower RC, Rowinsky EK (1993) Hepatic metabolism and biliary excretion of Taxol in rats and humans. J Natl Cancer Inst Monogr 15:39–46
Ozols RF, Bookman MA, du Bois A, Pfisterer J, Reuss A, Young RC (2006) Intraperitoneal cisplatin therapy in ovarian cancer: comparison with standard intravenous carboplatin and paclitaxel. Gynecol Oncol 103(1):1–6
Panday VR, Huizing MT, Willemse PH, De Graeff A, ten Bokkel Huinink WW, Vermorken JB, Beijnen JH (1997) Hepatic metabolism of paclitaxel and its impact in patients with altered hepatic function. Semin Oncol 24(4 Suppl 11):S11-34–S11-38
Park JH, Chi SC, Lee WS, Lee WM, Koo YB, Yong CS, Choi HG, Woo JS (2009) Toxicity studies of cremophor-free paclitaxel solid dispersion formulated by a supercritical antisolvent process. Arch Pharm Res 32(1):139–148
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, Stuart G, Kaye S, Vergote I, Blom R, Grimshaw R, Atkinson RJ, Swenerton KD, Trope C, Nardi M, Kaern J, Tumolo S, Timmers P, Roy JA, Lhoas F, Lindvall B, Bacon M, Birt A, Andersen JE, Zee B, Paul J, Baron B, Pecorelli S (2000) Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst 92(9):699–708
Rahman A, Korzekwa KR, Grogan J, Gonzalez FJ, Harris JW (1994) Selective biotransformation of taxol to 6 alpha-hydroxytaxol by human cytochrome P450 2C8. Cancer Res 54(21):5543–5546
Schellens JH, Malingre MM, Kruijtzer CM, Bardelmeijer HA, van Tellingen O, Schinkel AH, Beijnen JH (2000) Modulation of oral bioavailability of anticancer drugs: from mouse to man. Eur J Pharm Sci 12(2):103–110
Sonnichsen DS, Relling MV (1994) Clinical pharmacokinetics of paclitaxel. Clin Pharmacokinet 27(4):256–269
Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, Borst P, Nooijen WJ, Beijnen JH, van Tellingen O (1997) Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci USA 94(5):2031–2035
Sun L, Shen J, Pang X, Lu L, Mao Y, Zeng M (2007) Phase I safety and pharmacokinetic study of magnesium isoglycyrrhizinate after single and multiple intravenous doses in Chinese healthy volunteers. J Clin Pharmacol 47(6):767–773
Terauchi F, Moritake T, Yamamoto Y, Ogura H (2002) Combination chemotherapy with paclitaxel and intraperitoneal cisplatin for ovarian cancer with disseminated lesions in the peritoneum and the diaphragm. Int J Clin Oncol 7(6):356–360
van Rossum TG, Vulto AG, Hop WC, Brouwer JT, Niesters HG, Schalm SW (1999) Intravenous glycyrrhizin for the treatment of chronic hepatitis C: a double-blind, randomized, placebo-controlled phase I/II trial. J Gastroenterol Hepatol 14(11):1093–1099
Veldt BJ, Hansen BE, Ikeda K, Verhey E, Suzuki H, Schalm SW (2006) Long-term clinical outcome and effect of glycyrrhizin in 1093 chronic hepatitis C patients with non-response or relapse to interferon. Scand J Gastroenterol 41(9):1087–1094
Walle T, Walle UK, Kumar GN, Bhalla KN (1995) Taxol metabolism and disposition in cancer patients. Drug Metab Dispos 23(4):506–512
Willey TA, Bekos EJ, Gaver RC, Duncan GF, Tay LK, Beijnen JH, Farmen RH (1993) High-performance liquid chromatographic procedure for the quantitative determination of paclitaxel (Taxol) in human plasma. J Chromatogr 621(2):231–238
Wu X, Zhang L, Gurley E, Studer E, Shang J, Wang T, Wang C, Yan M, Jiang Z, Hylemon PB, Sanyal AJ, Pandak WM Jr, Zhou H (2008) Prevention of free fatty acid-induced hepatic lipotoxicity by 18beta-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology 47(6):1905–1915
Acknowledgments
We offer our sincere thanks to the volunteers for their commitment and patience during the study. We are grateful for the financial support from Department of Health of Sichuan Province (No. 110437).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, K.J., Chen, W.Y., Chen, X. et al. Increased elimination of paclitaxel by magnesium isoglycyrrhizinate in epithelial ovarian cancer patients treated with paclitaxel plus cisplatin: a pilot clinical study. Eur J Drug Metab Pharmacokinet 39, 25–31 (2014). https://doi.org/10.1007/s13318-013-0136-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-013-0136-y