Abstract
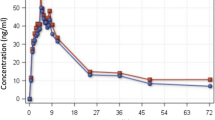
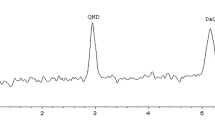
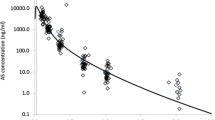
The effect of concurrent administration of a novel phytomedicine, NIPRD-AM1 used for the treatment of malaria on the pharmacokinetics of metronidazole was investigated in healthy volunteers. The study was a completely randomized one, crossover involving administration of single dose metronidazole tablets (200 mg × 2) concomitantly with NIPRD-AM1 capsules (250 mg × 2) to 11 healthy volunteers. Blood samples were collected before and at pre-determined time intervals following administration of the drugs. Serum concentrations of the unchanged metronidazole were analyzed using a modified simple and sensitive reversed phase high performance liquid chromatography (HPLC) method. The method showed good precision for metronidazole with coefficient of variation less than 10%. The Pharmacokinetic parameters (AUC, C max, and T max) were generated using GraphPad Prism software version 2. The derived pharmacokinetic parameters (AUC, C max) following the administration of metronidazole alone and co-administration with NIPRD-AM1 were 76.12 μg/ml per hour, 7.94 μg/ml and 73.52 μg/ml per hour, 7.83 μg/ml, respectively. This differences were not statistically significant (P < 0.05) and the relative bioavailability was found to be about 96%. The comparable relative bioavailabilty value obtained shows that there is little or no interaction between NIPRD-AM1 and metronidazole. The findings, therefore, showed that metronidazole can be administered with the phytomedicine NIPRD-AM1 without any significant effect on the pharmacokinetic profiles of metronidazole.


Similar content being viewed by others
References
Benvit-vical F, Valentin A, Cournac V, Pelisser Y, Mallie M, Bastide J (1998) Antiplasmodial activity of stem and root extracts of Nauclea latifolia S.M. (Rubiaceae). J Ethnopharmacol 61:173–178
British Pharmacopoeia (2004) vol II, pp 1306–1307
Daziel JK (1957) The useful plants of West Tropical Africa, 2nd edn. Crown agents, London, p 361
Duchateau A (1998) Posicor: Veni, Vidi, and Gone. Pharm Weekblad 133:1294–1295
Eisenberg DM, Davis RB, Ettner SL et al (1998) Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA 280:1569–1575
FDA (1992) Statistical procedures for bioequivalence studies using a standard two-treatment crossover design. Division of Bioequivalence, Office of Generic Drugs, Rockville, MD, USA
Galmier MJ, Frasey AM, Bastide M, Beyssac E, Petit J, Aiache C, Lartigue-Mattei (1998) Simple and sensitive method for determination of metronidazole in human serum by high-performance liquid chromatography. J Chromatogr B 720:239–243
Gamaniel K, Wambebe C, Amupitan J, Hussaini IM, Amos S, Awodogan A, Dunah AW, Ekuta JE, Akeju MO, Usman H, Emwerem M (1997) Active column fractions of Nauclea latifolia on plasmodium berghei and rabbit ileum. J Pharm Res Dev 2(1):44–47
Lazarou J, Pomeranz BH, Corey PN (1998) Incidence of adverse drug reaction in hospitalized patients: a meta-analysis of prospective studies. JAMA 279:1200–1205
Obodozie O, Mustapha K, Inyang U (2004) Alteration of oral salivary pharmacokinetics of paracetamol by an investigational anti-malarial phytomedicine, in healthy human volunteers. Eur J Drug Metabol Pharmacokinet 29(3):193–197
Obodozie OG, Mustapha KB, Taibat O, Garba M (2006) The effect of phytomedicines on the single dose pharmacokinetics of metronidazole and chloroquine. In: American Society of Pharmacognosy 47th annual meeting, Arlington, VA, 5–9 August, p 243
Ralph ED (1983) Clinical pharmacokinetics of metronidazole. Clin Pharmacokinet 8:43–62
Rauws EAJ, Van der Hulst RW (1995) Current guide lines for the eradication of Helicobacter pylori in peptic ulcer disease. Drugs 50:984–990
Acknowledgment
This study was funded by a research grant from the management committee of NIPRD, Idu, Abuja, Nigeria. Preparation of NIPRD/AM1 extract was by the Department of Medicinal Plant Research and Traditional Medicine, while the capsules are formulated by the Pharmaceutical Technology Department.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obodozie, O.O., Ebeshi, B.U., Mustapha, K.B. et al. The effects of an investigational antimalarial agent, NIPRD-AM1 on the single dose pharmacokinetics of metronidazole in healthy human volunteers. Eur J Drug Metab Pharmacokinet 35, 103–108 (2011). https://doi.org/10.1007/s13318-010-0012-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-010-0012-y