Abstract
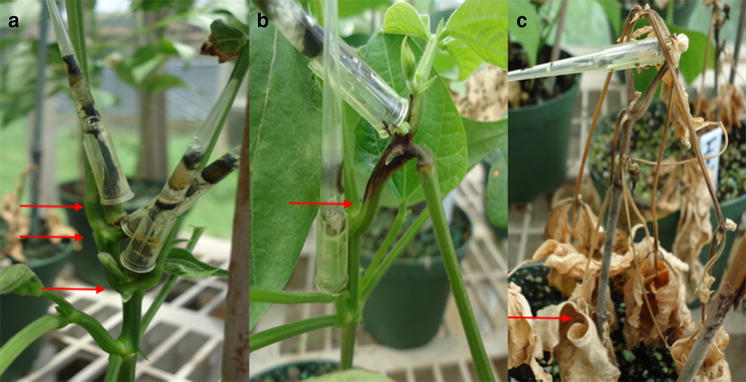
Thirteen jute genotypes were evaluated for resistance against Macrophomina phaseolina (Mp) in the sick plot, and six of them were identified for stable differential disease reactions. We tested whether other screening methods could produce similar results in these host genotypes. An incubation temperature of 35 °C was optimum for Mp sclerotia germination (90.8%), and it was chosen for experiments involving sclerotia as inoculum. Infestation with 250 or more Mp sclerotia/g seed induced morbidity in ~ 40% seedlings in vitro. Inoculation at the root and leaf could not differentiate between susceptible (JRO 524) and resistant (JRO 204) cultivars. Infesting both growth media and seed with Mp sclerotia induced early (15 days post-inoculation, DPI) expression of disease symptoms. However, the host reactions were in great variation with those from the field screening. Mp inoculum consisting of growing mycelial tips was more aggressive producing 6.4 cm long stem lesion at 4 DPI as compared to 3.9 cm by 7-day-old inoculum. Inoculating at the middle of the main stem produced the largest lesion (5.7 cm long) compared to the basal (3.3 cm) and the tip (1.3 cm) inoculations 6 DPI. Plants at ≥ 70 days after sowing (DAS) produced larger stem lesions than younger plants. This technique grouped test genotypes similar to sick plot experiments, with minor variations. Stem inoculation method may be adopted for large-scale screening and also confirming the resistance reaction of jute towards Mp infection.






Similar content being viewed by others
Change history
19 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s13313-021-00810-3
References
Adamowski M, Friml J (2015) PIN–dependent auxin transport: action, regulation, and evolution. Plant Cell 27:20–32
Baird RE, Watson CE, Scruggs M (2003) Relative longevity of Macrophomina phaseolina and associated mycobiota on residual soybean roots in soil. Plant Dis 87:563–566
Billones-Baaijens R, Jones EE, Ridgway HJ, Jaspers MV (2013) Virulence affected by assay parameters during grapevine pathogenicity studies with Botryosphaeriaceae nursery isolates. Plant Pathol 62:1214–1225
Chowdhury S, Basu A, Roy Chaudhuri T, Kundu S (2014) In–vitro characterization of the behaviour of Macrophomina phaseolina (Tassi) Goid at the rhizosphere and during early infection of roots of resistant and susceptible varieties of sesame. Eur J Plant Pathol 138:361–375
Coser SM, Chowda Reddy RV, Zhang J, Mueller DS, Mengistu A, Wise KA, Allen TW, Singh A, Singh AK (2017) Genetic architecture of charcoal rot (Macrophomina phaseolina) resistance in soybean revealed using a diverse panel. Front Plant Sci 8:1626. https://doi.org/10.3389/fpls.2017.01626
Das IK, Indira S, Annapurna A, Prabhakar, Seetharama N (2008) Biocontrol of charcoal rot in sorghum by fluorescent pseudomonads associated with the rhizosphere. Crop Prot 27:1407–1414
Grezes-Besset B, Lucante N, Kelechian V, Dargent R, Muller H (1996) Evaluation of castor bean resistance to sclerotial wilt disease caused by Macrophomina phaseolina. Plant Dis 80:842–846
Gupta GK, Sharma SK, Ramteke R (2012) Biology, epidemiology and management of the pathogenic fungus Macrophomina phaseolina (Tassi) Goid with special reference to charcoal rot of soybean (Glycine max (L.) Merrill). J Phytopathol 160:167–180
Kebrom TH (2017) A growing stem inhibits bud outgrowth—the overlooked theory of apical dominance. Front Plant Sci 8:1874. https://doi.org/10.3389/fpls.2017.01874
Khan KA, Shoaib A, Arshad AZ, Basit A, Hussain M (2018) Macrophomina phaseolina alters the biochemical pathway in Vigna radiata chastened by Zn2+ and FYM to improve plant growth. Journal of Plant Interactions 13:131–140
Mah KM, Uppalapati SR, Tang Y, Allen S, Shuai B (2012) Gene expression profiling of Macrophomina phaseolina infected Medicago truncatula roots reveals a role for auxin in plant tolerance against the charcoal rot pathogen. Physiol Mol Plant Pathol 79:21–30
Mandal RK, Sarkar S, Saha MN (2000) Field evaluation of white jute (Corchorus capsularis L.) germplasm against Macrophomina phaseolina (Tassi) Goid under Sorbhog condition. Environ Ecol 18:814–818
Mawar R, Lodha S (2009) Prior weakening of Macrophomina phaseolina and Fusarium propagules for enhancing efficiency of Brassica amendments. Crop Prot 28:812–817
Meshram JH, Palit P (2013) On the role of cell wall lignin in determining the fineness of jute fibre. Acta Physiol Plant 35:1565–1578
Mihali JD, Alcorn SM (1982) Quantitative recovery of Macrophomina phaseolina sclerotia from soil. Plant Dis 66:662–663
Mihaljcenic, M (1978) Choosing a suitable technique for screening sunflower for resistance to charcoal rot in climatic condition of Yugoslavia. Proceedings of 11th International Sunflower Conference, Minneapolis, pp 258–264
Nene YL, Haware MP, Reddy MV (1981) Chickpea disease resistance screening techniques. Information Bulletin, ICRISAT (10), Hyderabad, pp 10
Palit P, Meshram JH (2004) Physiological characterization of a phenotypically distinct jute (Corchorus olitorius) genotype. Plant Genetic Resources 2:175–180
Pickel B, Dai N, Maymon M, Elazar M, Tanami Z, Frenkel O, Toamy MA, Mor N, Freeman S (2020) Development of a reliable screening technique for determining tolerance to Macrophomina phaseolina in strawberry. Eur J Plant Pathol 157:707–718
Roy A, De RK, Ghosh SK (2008) Diseases of bast fibre crops and their management. In: Karmakar PG, Hazra SK, Ramasubramanian T, Mandal RK, Sinha MK, Sen HS (eds) Jute and Allied Fibre Updates. Central Research Institute for Jute and Allied Fibres, Kolkata, pp 217–241
Schroeder MM, Lai Y, Shirai M, Alsalek N, Tsuchiya T, Roberts P, Eulgem T (2019) A novel Arabidopsis pathosystem reveals cooperation of multiple hormonal response-pathways in host resistance against the global crop destroyer Macrophomina phaseolina. Sci Rep 9:20083
Shekhar M, Dutta R, Kumar S (2010) Potential bio–control agent for management of post–flowering stalk rot complex and maize plant health. Archives of Phytopathology and Plant Protection 14:1392–1395
Siddique S, Shoaib A, Khan SN, Mohy–ud–din A, (2021) Screening and histopathological characterization of sunflower germplasm for resistance to Macrophomina phaseolina. Mycologia 113:92–107
Srivastava RK, Singh RK, Kumar N, Singh S (2010) Management of Macrophomina disease complex in jute (Corchorus olitorius) by Trichoderma viride. J Biol Control 24:77–79
Twizeyimana M, Hill CB, Pawlowski M, Paul C, Hartman GL (2012) A cut–stem inoculation technique to evaluate soybean for resistance to Macrophomina phaseolina. Plant Dis 96:1210–1215
Van der Plank JE (1963) Plant diseases: epidemics and control. Academic Press, New York
Viana FMP, de Souza NL (2002) Efeito da interação temperatura–tensão de água sobre germinação de microescleródios de Macrophomina phaseolina. Fitopatol Bras 27:268–272
Zveibil A, Mor N, Gnayem N, Freeman S (2012) Survival, host–pathogen interaction, and management of Macrophomina phaseolina on strawberry in Israel. Plant Dis 96:265–272
Acknowledgements
We are thankful to Dr. Suman Roy, ICAR–CRIJAF, for critical reading of the manuscript and helpful discussion. Excellent technical help received from Mr. S. Nandi is duly acknowledged.
Funding
The authors received financial support from the Director, ICAR–CRIJAF, through two projects (JM 8.5 and JB 10.1). The work is partially funded by the ICAR, New Delhi, through a TMJ project.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original online version of this article was revised: In this article the legend 'JRO 204' for Fig. 6 was inadvertently omitted after 'OIN-125'; the figure has been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mandal, K., Kumar, A.A. & Ghosh, D. Development of screening techniques to evaluate response of jute (Corchorus olitorius) to Macrophomina phaseolina. Australasian Plant Pathol. 50, 621–630 (2021). https://doi.org/10.1007/s13313-021-00798-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-021-00798-w