Abstract
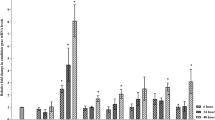
Antifungalmycin N2 (3-methyl-3,5-amino-4-vinyl-2-pyrone, C6H7O2N), was a novel and broad-spectrum antifungal metabolite produced by Streptomyces sp. N2. In the present work, antifungalmycin N2 was evaluated its induction of resistance against Rhizoctonia solani in rice seedling by measuring the defense-related physiological and biochemical parameters, such as the production of reactive oxygen species (ROS), the enzymatic activities involved in antioxidant systems (superoxide dismutase, SOD; catalase, CAT; peroxidase, POD), phenolics metabolism (phenylalanine ammonia-lyase, PAL; polyphenol oxidase, PPO), and pathogenesis-related proteins (β-1,3-glucanase and chitinase). It was found that the percentage disease index (PDI) of the R. solani-inoculated rice reduced from 65.21% to 26.02% when treated with 5.77 μg/ml antifungalmycin N2. Further results showed that the rice seedling could trigger its defense responses to R. solani infection by sharply accelerating the accumulation of ROS such as ·O2¬ and H2O2. But when the R. solani-inoculated rice was treated with antifungalmycin N2, the antioxidant SOD, CAT, and POD were significantly induced during the whole period of post inoculation (24–96 h), and consequently resulted in a sharp decline of ·O2¬ and H2O2 in rice seedlings. Compared with the R. solani-inoculated rice, the enzymatic activities of PPO, PAL, β-1,3-glucanase, and chitinase were significantly enhanced in the rice treated with antifungalmycin N2 plus R. solani. In conclusion, the above results suggested that antifungalmycin N2 could induce the resistance against R. solani in rice seedling by stimulating a series of defense responses, which would provide a comprehensive insight into the biocontrol function beyond its direct antagonistic activity against plant fungal pathogens.





Similar content being viewed by others
References
Armin SM, Avat S, Mohammad P (2014) Reactive oxygen species (ROS) generation and detoxifying in plants. J Plant Nutr 37:1573–1585
Basu A, Chowdhury S, Ray Chaudhuri T, Kundu S (2016) Differential behaviour of sheath blight pathogen Rhizoctonia solani in tolerant and susceptible rice varieties before and during infection. Plant Pathol 8:1333–1346
Cai KZ, Gao D, Luo SM, Zeng RS (2010) Physiological and cytological mechanisms of silicon-induced resistance in rice against blast disease. Physiol Plant 134:324–333
Chakraborty S, Newton AC (2011) Climate change, plant diseases and food security: an overview. Plant Pathol 60:2–14
Edreva A (2005) Pathogenesis-related proteins: research progress in the last 15 years. Gen Appl Plant Physiol 31:105–124
Feng SJ, Shu CW, Wang CJZ, Jiang SF (2017) Survival of Rhizoctonia solani AG-1 IA, the causal agent of rice sheath blight, under different environmental conditions. J Phytopathol 165:44–522
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28:1091–1101
González-Pérez S, Gutiérrez J, García-García F, Osuna D, Dopazo J, Lorenzo Ó, Revuelta JL, Arellano JB (2011) Early transcriptional defense responses in arabidopsis cell suspension culture under high-light conditions. Plant Physiol 156:1439–1456
Groth DE (2008) Effects of cultivar resistance and single fungicide application on rice sheath blight, yield, and quality. Crop Prot 27:1125–1130
Hahn M (2014) The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J Chem Biol 7:133–141
Harman GE (2006) Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96:190–194
Hyun MW, Yun YH, Kim JY, Kim SH (2011) Fungal and plant phenylalanine ammonia-lyase. Mycobiology 39:257–265
Ke D, Sun GC, Wang ZX (2007) Effects of superoxide radicals on ACC synthase activity in chilling-stressed etiolated mungbean seedlings. Plant Growth Regul 51:83–91
Ke Y, Deng H, Wang S (2017) Advances in understanding broad-spectrum resistance to pathogens in rice. Plant J 90:738–748
Khaledi N, Taheri P, Falahati-Rastegar M (2016) Reactive oxygen species and antioxidant system responses in wheat cultivars during interaction with Fusarium species. Australas Plant Pathol 45:653–670
Kinkel LL, Schlatter DC, Bakker MG, Arenz BE (2012) Streptomyces competition and co-evolution in relation to plant disease suppression. Res Microbiol 163:490–499
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Law JW, Ser HL, Khan TM, Chuah LH (2017) The potential of Streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front Microbiol 8:3
Leadbeater A (2015) Recent developments and challenges in chemical disease control. Plant Prot Sci 51:163–169
McSpadden Gardener BB, Driks A (2004) Overview of the nature and application of biocontrol microbes: Bacillus spp. Phytopathology 94:1244
Mi LN, Niu XJ, Lu MQ, Ma JL, Wu JD, Zhou XQ (2014) Phosphine-induced physiological and biochemical response in rice seedlings. Chemosphere 100:77–82
Molinier J, Ries G, Zipfel C, Hohn B (2006) Transgeneration memory of stress in plants. Nature 442:1046–1049
Mukherjee N, Kundu B (2010) Antifungal activities of some phenolics and related compounds to three fungal plant pathogens. J Phytopathol 78:89–92
Nadarajah KK (2017) Induction of systemic resistance for sisease suppression. In: Abdullah S, Chai-Ling H, Wagstaff C (eds) Crop improvement. Springer, Cham, pp 335–357
Narayanasamy P (2013) Mechanisms of action of fungal biological control agents. In: Biological management of diseases of crops. Progress in biological control, vol 14. Springer, Netherlands, pp 99–200
O’Brien JA, Daudi A, Butt VS, Paul Bolwell G (2012) Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236:765–779
Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker PAHM (2014) Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol 52:347–375
Przemysław WOJTASZEK (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J 322:681–692
Qi JS, Wang JL, Gong ZZ, Zhou JM (2017) Apoplastic ROS signaling in plant immunity. Curr Opin Plant Biol 38:92–100
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Rojas C, Muthappa SK, Tzin V, Mysore KS (2014) Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front Plant Sci 5:17
Shoresh M, Harman GE, Mastouri F (2010) Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol 48:1–23
Singh R, Soni SK, Awasthi A, Kalra A (2012) Evaluation of vermicompost doses for management of root-rot disease complex in Coleus forskohlii, under organic field conditions. Australas Plant Pathol 41:397–403
Strange RN, Scott PR (2005) Plant disease: a threat to global food security. Annu Rev Phytopathol 43:83–116
Suzuki N, Koussevitzky S, Mittler R, Miller G (2011) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Thomashow LS (2016) Induced systemic resistance: a delicate balance. Environ Microbiol Rep 8:560–563
Van Loon LC, Rep M, Pieterse CM (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44:135–162
Vanitha SC, Niranjana SR, Umesha S (2010) Role of phenylalanine ammonia lyase and polyphenol oxidase in host resistance to bacterial wilt of tomato. J Phytopathol 157:552–557
Weller DM (2007) Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology 97:250–256
Wu ZM, Yang Y, Li KT (2019) Antagonistic activity of a novel antifungalmycin N2 from Streptomyces sp. N2 and its biocontrol efficacy against Rhizoctonia solani. FEMS Microbiol Lett 366:fnz018
Xu B, Chen W, Wu ZM, Long Y, Li KT (2015) A novel and effective Streptomyces sp. N2 against various phytopathogenic Fungi. Appl Biochem Biotechnol 177:1338–1347
Yin D, Deng X, Chet I, Song R (2014) Physiological responses of Pinus sylvestris var. Mongolica seedlings to the interaction between Suillus luteus and Trichoderma virens. Curr Microbiol 69:334–342
Yoruk R, Marshall MR (2003) Physicochemical properties and function of plant polyphenol oxidase: a review. J Food Biochem 27:361–422
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31760546), and the Training Program for Young Scientists of Jiangxi Provincial Department of Science and Technology (20142BCB23025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, Sw., Yang, Y., Wu, Zm. et al. Induced defense responses against Rhizoctonia solani in rice seedling by a novel antifungalmycin N2 from Streptomyces sp. N2. Australasian Plant Pathol. 49, 267–276 (2020). https://doi.org/10.1007/s13313-020-00703-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-020-00703-x