Abstract
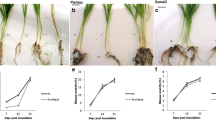
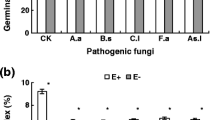
Susceptibility of sorghum seedlings to infection by Sporisorium sorghi, the fungus causing covered kernel smut (CKS) disease, is limited to the period between the sowing of the seed and emergence of the seedling. Microscopy and PCR diagnosis of 7-day-old infected seedlings detected hyphae of S. sorghi in plumule and mesocotyl tissue but not in coleoptile and radicle tissue. Percent infected seedling plumule and mesocotyl tissue detected by microscopy and PCR corresponded well to field resistance classes for CKS in mature panicles of both sorghums tested with Giza-15 being highly susceptible and hybrid Shandweel-305 being resistant. Bulk extracts of phenolics and antioxidants from seedling plumule and mesocotyl tissues of hybrid Shandweel-305 cv significantly inhibited the in vitro mycelial growth of S. sorghi more than those from Giza-15 cv. Moreover, bulk extracts of plumule and mesocotyl tissue of seedlings grown from seed inoculated with S. sorghi teliospores contained higher phenolic and antioxidant content in the resistant hybrid (Shandweel-305) than the highly susceptible cultivar (Giza-15). Similarly, bulk extracts of plumule and mesocotyl tissue of 7-day-old seedlings grown from seed inoculated with S. sorghi teliospores contained higher phenolic and antioxidant content in the resistant hybrid (Shandweel-305) than the highly susceptible cultivar (Giza-15). Ferulic, isorhamnetin and kaempferol acids were detected only in plumule and mesocotyl tissue of resistant hybrid Shandweel-305, whereas ellagic, fumaric and vanillic acids were detected only in highly susceptible Giza-15 cv. Ferulic acid added to malt dextrose broth medium significantly inhibited in vitro teliospore germination and mycelial growth of S. sorghi, especially when added at the highest concentrations. This research established that the mode of seedling infection of sorghum by S. sorghi is almost exclusively through the plumule and mesocotyl tissue. Within these tissues, the presence of the pathogen can induce host production of phenolics, including the fungitoxic ferulic acid and antioxidants at higher concentrations within the resistant sorghum than in the highly susceptible cultivar.







Similar content being viewed by others
References
Abera M, Alemayehu G (2012) Evaluation of improved and local/landrace/sorghum varieties for covered kernel smut. Arch Phytopathol Plant Protect 45(6):717–723
Akhtar MA, Sarwar M, Hakro AA, Khan NA (1989) Evaluation of sorghum cultivars to covered kernel smut. Pak J Agric Res 10(1):45–48
Anjum T, Fatima S, Amjad S (2012) Physiological changes in wheat during development of loose smut. Trop Plant Pathol 37(2):102–107
Arici SE, Kafkas E, Kaymak S, Koc NK (2014) Phenolic compounds of apple cultivars resistant or susceptible to Venturia inaequalis. Pharm Biol 52(7):904–908
Arora YK, Wagle DS (1985) Interrelationship between peroxidase, polyphenol oxidase activities, and phenolic content of wheat for resistance to loose smut. Biochem Physiol Pflanz 180:75–78
Assabgui RA, Reid LM, Hamilton RI, Arnason JT (1993) Correlation of kernel (E)- Ferulic acid content of maize resistance to Fusarium graminearum. Phytopathology 83:949–953
Bai J, Pan S, Qi P (1980) The seedling test as a method of forecasting the infection of head smut in sorghum with notes on the process of systemic infection. Acta Phytopathologica Sinica 10:37–42
Botros SE (1993) Studies on kernel smut of sorghum in Upper Egypt. Ph.D. thesis, Faculty of Agriculture, Assiut University, Egypt, 129 pp
Bray HG, Thorpe WV (1954) Analysis of phenolic compounds of interest in metabolism. Methods Biochem Anal 1:27–52
Claflin LE, Ramundo BA (1996) Evolution of all diseases and insect sorghum germplasm for susceptibility to covered kernel smut. Phytopathology 86:S63 (Abstract)
Dicko MH, Gruppen H, Barro C, Traore AS, van Berkel WJH, Voragen AGJ (2005) Impact of phenolic compounds and related enzymes in sorghum varieties for resistance and susceptibility to biotic and abiotic stresses. J Chem Ecol 31(11):2671–2688
El-Helaly AF, Ibrahim IA (1957) Host-parasite relationship of Sphacelotheca sorghi on sorghum. Phytopathology 47:620–623
El-sayed ABB, Sadoma MT, Awad HMF (2010) Reaction of some grain sorghum cultivars to downy mildew disease infection caused by Peronosclerospora sorghi. J Plant Prot and Pathol, Mansoura Univ, Egypt 1(12):949–957
El-Sherbeni AE, El-Zahaby HM, Kishk AA, Awad HMF, Aml EA (2008) Biochemical changes due to downy mildew disease resistance caused by Peronosclerospora sorghi in some Egyptian grain sorghum cultivars. In: Proceedings of the first international conference on environmental studies and research. Environmental Studies and Research Institute, Minufiya University, Sadat Branch, Egypt, 7–9 April 2008, pp 10–18
Faris JA, Reed GM (1925) Modes of infection of sorghums by loose kernel smut. Mycologia 17(2):51–67
French RC (1961) Stimulation of uredospore germination in wheat stem rust by terpenes and related compounds. Bot Gaz 122(3):194–198
Gazar AA (1985) Integrated control of sorghum Kernel smut in relation to environmental conditions. In: Proceedings of Egypt. Bot. Soc. 4, 1985, Ismailia conference, pp 1081–1100
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research, 2nd edn. Willey, New York
Gwary DM, Bdliya BS, Bwatanglang N (2009) Integration of fungicides, crop varieties and sowing dates for the management of sorghum smuts in Nigerian Savanna. Arch Phytopathol Plant Protect 42(10):988–999
Harmas H, Terba M (1984) Metabolism of phenolic compounds in healthy and brown rust infected barley and wheat varieties. Phytopathol Z 111:283–296
Huang LD, Backhouse D (2005) Induction of defence responses in root and mesocotyls of sorghum seedlings by inoculation with Fusarium thapsinum and F. proliferatum, wounding and light. J Pytopathol 123:522–529
Jabeen N, Ahmed N, Ghani YM, Sofi AP (2009) Role of phenolic compounds in resistance to chilli wilt. Commun Biometry Crop Sci 4(2):52–61
Jambunathan R, Butler LG, Bandyopadhyay R, Mughogho LK (1986) Polyphenol concentrations in grain, leaf, and callus tissues of mold-susceptible and mold-resistant sorghum cultivars. J Agric Food Chem 34:425–429
Kalappanavar IK, Hiremath RV (2000) Biochemical factors for multiple resistance to foliar disease of sorghum. Madras Agric J 87:66–70
Kaur M, Deshpande KB (1980) Effect of different phenolic compounds on spore germination of Sphacelotheca sorghi (Link) Clint. and S. reiliana (Kuhn) Clint. Acta Bot Ind 8(2):243–246
Khaleeque MI, Alam A, Imam-al-Hag M, Ahmed M (1995) Reaction of ten sorghum (Sorghum bicolor L.) cultivars against grain smut (Sphacelotheca sorghi (Link) Clint.) under semi-arid conditions of Bahavalpur. Pak J Phytopathol 7(1):90–91
Khatun S, Cakilcioglu U, Chakrabarti M, Ojha S, Chatterjee NC (2011) Biochemical defense against die-back disease of a traditional medicinal plant Mimusops elengi Linn. Eur J Med Plant 1:40–49
Kil HY, Seong ES, Ghimire BK, Chung IM, Kwon SS, Goh EJ, Heo K, Kim MJ, Lim JD, Lee D et al (2009) Antioxidant and antimicrobial activities of crude sorghum extract. Food Chem 115:1234–1239
Kollmorgen JF, Ballinger DJ (1987) Detection and morphology of hyphae of common bunt fungi (Tilletia leavis and T. tritici) in wheat seedlings. Trans Br Mycol Soc 88(4):555–559
Kollo AI (2000) Compendium of Sorghum diseases. In: Frederiksen RA, Odvody GN (eds) Long smut. American Phytopathological Society, St. Paul, pp 22–23
Kulbat K (2016) The role of phenolic compounds in plant resistance. Biotechnol Food Sci 80(2):97–108
Lindsay P (2016) Screening of sorghum (Sorghum bicolor) genotypes for resistance to covered kernel smut (Sporisorium sorghi) disease. A research project of the degree of Bachelor of Science in Agronomy. Department of Agronomy, Faculty of Natural Resources Management and Agriculture, Midlands State University, Gweru, Zimbabwe, 33 pp
Mahadik VB, Mali NS (2018) Antioxidant activity in safflower (Carthamus tinctorius L.) cultivars under the pathogenesis of foliar fungal disease complex. Indian J Agric Res 52(1):76–80
Malaguti G (2003) Investigaciones sobre la pathogénesis del carbén del arroz. Agronomía Trop 53(4):70–77 (English abstract online)
Matyac CA (1985) Histological development of Sphacelotheca reiliana on Zea mays. Phytopathology 75:924–929
McKinney HH (1923) Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. J Agric Res 26(5):195–218
Mcknight T (1964) Studies on the fungus Sphacelotheca sorghi (Link) Clinton. Ph.D. thesis, The University of Queensland, UK, 171 pp
Mehrotra RS, Aggarwal A (2003) Plant pathology, 2nd edn. Tata McGraw-Hill Publishing Company Limited, New Delhi, 847 pp
Melchers LW, Hansing ED (1938) The influence of environmental conditions at planting time on sorghum kernel smut infection. Am J Bot 25:17–28
Melo GA, Shimizu MM, Mazzafera M (2006) Polyphenoloxidase activity in coffee leaves and its role in resistance against the coffee leaf miner and coffee leaf rust. Phytochemistry 67:277–285
Millhollon R (2000) Loose kernel smut for biocontrol of Sorghum halpense in Saccharum spp. hybrids. Weed Sci 48:645–652
Mirza MS, Hamid SJ, Hassan SF (1982) Resistance of sorghum varieties to covered smut. Pak J Agric Res 3:31–33
Moharam MHA, Leclerque A, Koch E (2012) Cultural characteristics of Sporisorium sorghi and detection of the pathogen in plant tissue by microscopy and polymerase chain reaction. Phytoparasitica 40(5):475–483
Moharam HAM, Stephan D, Koch E (2018) Evaluation of plant derived preparations and microorganisms as seeds treatments for control of covered kernel smut of sorghum (Sporisorium sorghi). J Plant Dis Prot 125(2):159–166
Mukherjee SP, Choudhuri MA (1983) Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58(2):166–170
Munkacsi AB, Stoxen S, May G (2007) Domestication of maize, sorghum, and sugarcane did not drive the divergence of their smut pathogens. Evolution 61:388–403
Naik ST, Anahosur KH, Hegde RK (1981) Role of sugars, phenols and amino acids in rust resistance in sorghum. Mysore J Agric Sci 15:282–288
Narayanasamy P (2005) Postharvest pathogens and disease management. Wiley, Hoboken, 578 pp
Nicholson RL, Kollipara SS, Vincent JR, Lyons PC, Cadena-Gomez G (1987) Phytoalexin synthesis by the sorghum mesocotyl in response to infection by pathogenic and nonpathogenic fungi. Proc Natl Acad Sci U S A 84:5520–5524
Nzioki HS, Claflin LE, Ramundo BA (2000) Evaluation of screening protocols to determine genetic variability of grain sorghum germplasm to Sporisorium sorghi under field and greenhouse conditions. J Pest Manag 46(2):91–95
Oleinik AA (1989) Source of resistance to Sphacelotheca sorghi selektsiya, agrotekhnika ekononika proizvodstra sorgo: sorniknauchngkh trudov. Zernagrad, USSR, 1989: 119–124 (c.f. Rev Plant Pathol 70:5625, 1991)
Patil BB, Sajjan AS, Patil SB, Gangashetty PI (2011) Effect of grain smut incidence on crop growth, seed yield and quality parameters of Rabi sorghum. Int J For Crop Improv 2(1):36–39
Popp W (1955) A comparative study of spore germination of Ustilago tritici and U. nuda. Phytopathology 45:585–590
Quiroga M, Guerrero C, Botella A, Barcelo A, Amaya I, Medina MI, Alonso FJ, de Forchetti SM, Tigier T, Valpuesta V (2000) A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol 122:1119–1127
Ramasamy P, Frederiksen RA, Prom LK, Magill CW (2007) Head smut. In: Thakur RP, BVS R, Mathur K (eds) Screening techniques for sorghum diseases, Information Bulletin No. 76. International Crops Research Institute for the Semi-Arid Tropics, Patancheru, pp 58, 92 pp–63
Sharma I, Kumari N, Sharma V (2014) Defense gene expression in Sorghum bicolor against Macrophomina phaseolina in leaves and roots of susceptible and resistant cultivars. J Plant Interact 9(1):315–323
Shimzu N, Hosogi N, Hyon GS, Jiang S, Inoue K, Park P (2006) Reactive oxygen species (ROS) generation and ROS induced lipid peroxidation are associated with plant membrane modifications in host cells in response to AK-toxin from Alternaria alternata Japanese pear pathotype. J Gen Plant Pathol 72:6–15
Soetan KO, Oyekunle MA, Aiyelaagbe OO, Fafunso MA (2006) Evaluation of the antimicrobial activity of saponins extract of Sorghum bicolor L. Moench. Afr J Biotechnol 5:2405–2407
Thakur RP (2007) Covered kernel smut. In: Thakur RP, BVS R, Mathur K (eds) Screening techniques for sorghum diseases, Information Bulletin No. 76. International Crops Research Institute for the Semi-Arid Tropics, Patancheru, pp 68, 92 pp–70
Ward EWB (1986) Biological mechanisms involved in resistance of plants of fungi. In: Baily JA (ed) Biology and molecular biology of plant-pathogen interactions. Springer, Berlin, pp 107–131
Wilson KSL (2011) Sorghum ratooning as an approach to manage covered kernel smut and the stem borer Chilo artellus. Ph.D. thesis, University of Grenmish, UK, 274 pp
Acknowledgments
The author is thankful to all members of the Experimental Farm, Faculty of Agriculture, Sohag University for financial support to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moharam, M.H.A., Hassan, M.A.A. Defense response of seedling plumule and mesocotyl of sorghum to infection by Sporisorium sorghi, causing covered kernel smut in relation to disease resistance classes. Australasian Plant Pathol. 49, 1–14 (2020). https://doi.org/10.1007/s13313-019-00670-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-019-00670-y