Abstract
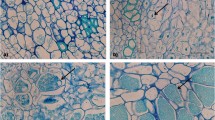
We developed an improved solution culture technique that enabled clear and comprehensive observation of dynamic root hair infection by Plasmodiophora brassicae. Brassica oleracea seedlings were sown directly into 10 mL centrifuge tubes containing half-strength Hoagland nutrient solution. Seedlings were fastened in 1 cm thick foam in the tube top and tubes were wrapped with black tape to block light and prevent algal growth. Maximal root hair infection was observed under conditions of pH 5.5, temperature of 20 to 25 °C, and an inoculation concentration of 1 × 107 spores/mL. Infection efficiency was obviously higher in fresh nutrient. Under optimum infection conditions, plasmodia were found in the root hair 4 days after inoculation (DAI), with some undergoing cleavage and developing into round zoosporangia 3 days later. At ten DAI, the number of root hairs containing zoosporangia per root reached 208, and the cytoplasm within the zoosporangia began to cleave and form incipient zoospores. Most zoosporangia released the zoospores and became diaphanous at ten DAI. Root hairs became swollen during the experimental trial and small galls appeared clearly on the root at 20 DAI. In addition, sectioned specimens showed that some cortical cells contained resting spores. We sequentially tested the germination of the resting spores in tubes from 1 to 11 DAI and the temporal dynamics of germination corresponding to the infection. Zoosporangia were found in epidermal cells at the same time as in the root hair.









Similar content being viewed by others
References
Asano T, Kageyama K, Hyakumachi M (1999) Surface disinfestation of resting spores of Plasmodiophora brassicae used to infect hairy roots of Brassica spp. Phytopathology 894:314–319
Asano T, Kageyama K, Hyakumachi M (2000) Germination of surface-disinfected resting spores of Plasmodiophora brassicae and their root hair infection in turnip hairy roots. Mycoscience 41:49–54
Asano T, Kageyama K (2006) Growth and movement of secondary plasmodia of Plasmodiophora brassicae in turnip suspension culture cells. Plant Pathol 55:145–151
Ayers GW (1944) Studies on the life history of the clubroot organism, Plasmodiophora brassicae. Can J Res (C) 32:143–149
Channon A, Flint AE, Hinton R (1964) A quantitative laboratory method for inoculating cabbage seedlings with Plasmodiophora brassicae Woron. Ann Appl Biol 541:71–76
Chiang M, Chiang B, Grant W (1977) Clubroot-resistance transferred to cabbage. Can Agric 224:23–24
Dobson R, Gabrielson R, Baker A (1982) Soil water matric potential requirements for root-hair and cortical infection of Chinese cabbage by Plasmodiophora brassicae. Phytopathology 7212:1598–1600
Dobson RL, Gabrielson R (1983) Role of primary and secondary zoospores of Plasmodiophora brassicae in the development of clubroot in Chinese cabbage. Phytopathology 734:559–561
Donald E, Porter I (2004) A sand solution culture technique used to observe the effect of calcium and pH on root hair and cortical stages of infection by Plasmodiophora brassicae. Australas Plant Pathol 334:585–589
Donald E, Jaudzems G, Porter I (2008) Pathology of cortical invasion by Plasmodiophora brassicae in clubroot resistant and susceptible Brassica oleracea hosts. Plant Pathol 572:201–209
Feng J, Hwang SF, Strelkov S (2012) Studies into primary and secondary infection processes by Plasmodiophora brassicae on canola. Plant Pathol. Online publication/2012/0327
Guo XH, Xiao CG (2002) Biological characteristic of Plasmodiophora brassicae. Mycosystema 214:585–589
Gustafsson M, Liljeroth E, Gunnarsson M, Lundborg T (1986) Effects of infection by Plasmodiophora brassicae on root anatomy of rape. J Phytopathol 117:144–151
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular. California Agricultural Experiment Station 347:23–32
Ingram D, Tommerup IC (1972) The life history of Plasmodiophora brassicae Woron. Proc R Soc London, Ser B 180:103–112
Kageyama K, Asano T (2009) Life cycle of Plasmodiophora brassicae. J Plant Growth Regul 283:203–211
Kim D, Oh J (1997) Incidence, pathogenicity of clubroot fungus Plasmodiophora brassicae and varietal resistance in Chinese cabbage. Korean J Plant Pathol 13:95–99
Macfarlane I (1958) A solution-culture technique for obtaining root-hair, or primary, infection by Plasmodiophora brassicae. J Gen Microbiol 183:720–732
Naiki T, Tanahashi K, Kageyama K (1984) The relationship between root hair infection with Plasmodiophora brassicae Wor. and subsequent club formation among cruciferous species. Ann Phytopathol Soc Jpn 50:211–215
Suzuki K, Matsumiya E, Ueno Y, Mizutani J (1992) Some properties of germination-stimulating factor from plants for resting spores of Plasmodiophora brassicae. Ann Phytopathol Soc Jpn 58:699–705
Tommerup IC, Ingram D (1971) The life-cycle of Plasmodiophora brassicae woron. in Brassica tissue cultures and in intact roots. New Phytol 702:327–332
Voorrips RE (1992) Root hair infection by Plasmodiophora brassicae in clubroot resistant and susceptible genotypes of Brassica oleracea, B. rapa and B. napus. Eur J Plant Pathol 986:361–368
Williams PH, Reddy MN, Strandberg JO (1969) Growth of noninfected and Plasmodiophora brassicae infected cabbage callus in culture. Can J Bot 47:1217–1221
Williams P, Aist SJ (1972) Response of cabbage root hairs to infection by Plasmodiophora brassicae. Can J Bot 491:41–47
Acknowledgments
We gratefully acknowledge funding from the special scientific project of the Ministry of Agriculture Public Welfare Industry (Agricultural), and the many people who have worked on or contributed to this study including student aides, technicians and colleagues; Dou Yan-xia for her thorough and capable technical assistance on all aspects of this work; Ma Guan-hua and Dong Guo-ju for additional technical support; and all the others without whom this study could not have been completed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, Hc., Chen, Gk., Liu, Cp. et al. An improved culture solution technique for Plasmodiophora brassicae infection and the dynamic infection in the root hair. Australasian Plant Pathol. 43, 53–60 (2014). https://doi.org/10.1007/s13313-013-0240-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-013-0240-0