Abstract
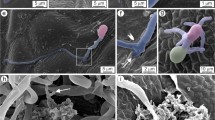
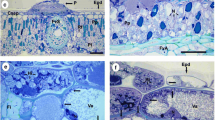
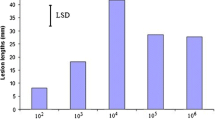
This study investigated the endophytic interactions between Botryosphaeriaceae pathogens and their woody hosts and the role of wounds in development of infection of green tissues. At 2 and 3 months after inoculating trunks of 2 year old grapevine plants with Neofusiciccum australe, N. luteum, N. parvum or Diplodia mutila, sap samples provided no evidence of pathogen propagules, indicating that the dieback symptoms observed at a distance from the lesions on inoculated sections of the trunks were not due to internally transmitted propagules. However, longitudinal sections cut from beyond trunk lesions showed hyphae of the four fungi grew within xylem vessels, which was consistent with endophytic fungal development observed in previous experiments. When conidia of N. luteum were inoculated onto green shoots and leaves of potted grapevines, which were wounded or non-wounded, and excised or attached to living plants, scanning electron microscope studies showed that conidial development was significantly affected by the condition of tissues. On detached and wounded leaf and shoot surfaces, most conidia had germinated by 3 h after inoculation and by 24 h had developed into networks of mycelium. On attached and wounded leaf and shoot surfaces it took 24 h for most conidia to germinate. However, when green tissues were attached and non-wounded, conidia did not adhere or germinate, all conidia being shed from the tissues within 24 h. These studies have provided explanations for phenomena observed during infection studies and have demonstrated that the pathogen-host interactions are complex, warranting further examination of the physiology of the interactions.







Similar content being viewed by others
References
Amiri A, Cholodowski D, Bompeix G (2005) Adhesion and germination of waterborne and airborne conidia of Penicillium expansum to apple and inert surfaces. Physiol Mol Plant Pathol 67(1):40–48
Amponsah NT (2011) Epidemiology of botryosphaeriaceous species associated with grapevine plants in New Zealand (PhD Thesis). Lincoln University, New Zealand.
Amponsah NT, Jones EE, Ridgway HJ, Jaspers MV (2009) Rainwater dispersal of Botryosphaeria conidia from infected grapevines. New Zealand Plant Protect 62:228–233
Amponsah NT, Jones EE, Ridgway HJ, Jaspers MV (2011a) Identification, potential inoculum sources and pathogenicity of botryosphaeriaceous species associated with grapevine dieback disease in New Zealand. Eur J Plant Pathol 131:467–482
Amponsah NT, Jones EE, Ridgway HJ, Jaspers MV (2011b) Susceptibility of grapevine tissues to Neofusicoccum luteum conidial infection. Plant Pathol. doi:10.1111/j.1365-3059.2011.02548.x
Baskarathevan J, Jaspers MV, Jones EE, Ridgway HJ (2011) Incidence and distribution of botryosphaeriaceous species in New Zealand vineyards. Eur J Plant Pathol. doi:10.1007/s10658-011-9900-5
Beckett A, Tatnell JA, Taylor N (1990) Adhesion and pre-invasion behaviour of urediniospores of Uromyces viciae fabae during germination on host and synthetic surfaces. Mycol Res 94:865–875
Bensalem-fnayou A, Jellouli N, Bouamama B, Mliki A, Ghorbel A (2009) Investigations on the leaf surface ultrastructure in grapevine (Vitis vinifera) by scanning microscopy. Scanning 31(3):127–131
Castillo-Pando M, Somers A, Green CD, Priest M, Sriskanthades M (2001) Fungi associated with dieback of Semillon grapevines in the Hunter Valley of New South Wales. Australas Plant Pathol 30(1):59–63
Collins TJ, Moerschbacher BM, Read ND (2001) Synergistic induction of wheat stem rust appressoria by chemical and topographical signals. Physiol Mol Plant Pathol 58:259–266
Droby S, Eick A, Macarisin D, Cohen L, Rafael G, Stange R, McColum G, Dudai N, Nasser A, Wisniewski M, Shapira R (2008) Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol Tech 49:386–396
Eckert JW, Ratnayake M (1994) Role of volatile compounds from wounded oranges in induction of germination of Penicillium digitatum conidia. Phytopathology 84:746–50
Eigenbrode SD, Jetter R (2002) Attachment to plant surface waxes by insect predator. J Integr Comp Biol 42:1091–1099
Filonow AB (2004) Adhesion of decay-causing fungal conidia in wounds of Malus × domestica ‘Golden Delicious’ apple fruit is influenced by wound age. Can J Bot 82(2):265–272
French RC (1992) Volatile chemical germination stimulators of rust and othe fungal spores. Mycologia 84:277–288
Garber RH, Houston BR (1966) Penetration and development of Verticillium albo-atrum in cotton plant. Phytopathology 56:1121–26
Hedge Y, Kolattukudy PE (1997) Cuticular waxes relieve self inhibition of germination and appressorium formation by the conidia of Manaporthe grisea. Physiol Mol Plant Pathol 51:75–84
Johnson GI, Sangchote S (1994) Control of postharvest diseases of tropical fruits: Challenges for the 21st century. In: Champ BR, Highley E, Johnson G (eds) Postharvest handling of tropical fruits. ACIAR Proceedings 50:140–161
Jones MJ, Epstein L (1989) Adhesion of Nectria haematococca macroconidia. Physiol Mol Plant Pathol 35:453–461
Klosterman SJ, Atallah ZK, Vallad GE, Subbarao KV (2009) Diversity, pathogenicity, and management of Verticillium species. Annu Rev Phytopathol 47:39–62
Phillips AJL (1998) Botryosphaeria dothidea and other fungi associated with excoriose and dieback of grapevines in Portugal. J Phytopathol 146(7):327–332
Phillips AJL (2002) Botryosphaeria species associated with diseases of grapevines in Portugal. Phytopathology Mediterranea 41:3–18
Ruehle, G. D. (1964). A Strain of Alternaria citri Ellis and Pierce causing a leaf spot of rough Lemon in Florida. Phytopathology 27: 863–865
Saad JH, Hagedorn DJ (1969) Host parasite relations in the initiation and development of bean alternaria leaf spot. Phytopathology 59:1773–1774
Smith H, Wingfield MJ, Crous PW, Coutinho TA (1996) Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. South African J Bot 62:86–88
Taylor A, St. J, Hardy GE, Wood P, Burgess T (2005) Identification and pathogenicity of Botryosphaeria species associated with grapevine decline in Western Australia. Australas Plant Pathol 34(2):187–195
Thorne ET, Young BM, Young GM, Stevenson JF, Labavitch JM, Matthews MA (2006) The structure of xylem vessels in grapevine and a possible passive mechanism for the systemic spread of bacterial disease. Am J Bot 93:497–504
Tucker SL, Talbot NJ (2001) Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu Rev Phytopathol 39:385–417
Úrbez-Torres J, Gubler WD (2012) Botryosphaeria dieback: the current status of the Botryosphaeriaceae species infecting grapevines. Proceedings of the 8 th International Workshop on Grapevine Trunk Diseases, Valencia, Spain, 18–21 June 2012: p 17
Úrbez-Torres J, Gubler WD (2009) Pathogenicity of Botryosphaeriaceae species isolated from grapevine cankers in California. Plant Dis Res 93(6):584–592
Úrbez-Torres J, Gubler WD (2011) Susceptibility of grapevine pruning wounds to infection by Lasioplodia theobromae and Neofusicoccum parvum. Plant Pathol 60:261–270
Úrbez-Torres JR, Battany M, Bettiga LJ, Gispert C, McGourty G, Roncoroni J, Smith RJ, Verdegaal P, Gubler WD (2010) Botryosphaeriaceae species spore-trapping studies in California vineyards. Plant Dis 94(6):717–724
van Niekerk JM, Crous P, Fourie PH, Groenewald E, Halleen F (2002) Botryosphaeria canker and dieback of grapevines. Wynboer 158:16–19
van Niekerk JM, Crous PW, Groenewald JZ, Fourie PH, Halleen F (2004) DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 96(4):781–798
Wunderlich N, Ash GJ, Steel CC, Raman H, Savocchia S (2011) Association of Botryosphaeriaceae grapevine trunk disease fungi with the reproductive structures of Vitis vinifera. Vitis 50:89–96
Acknowledgments
We are grateful to New Zealand Wine-growers and Lincoln University for funding this research, to Neil Andrews for his shilled help with viewing the specimens with scanning electron microscopy and to Brent Richards of the Lincoln University for maintaining the grapevine plants during the course of the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amponsah, N.T., Jones, E.E., Ridgway, H.J. et al. Microscopy of some interactions between Botryosphaeriaceae species and grapevine tissues. Australasian Plant Pathol. 41, 665–673 (2012). https://doi.org/10.1007/s13313-012-0159-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-012-0159-x