Abstract
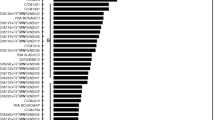
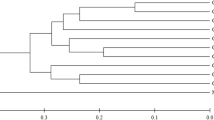
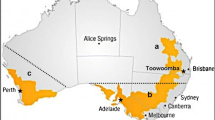
Australian and international chickpea (Cicer arietinum) cultivars and germplasm accessions, and wild annual Cicer spp. in the primary and secondary gene pools, were assessed in glasshouse experiments for levels of resistance to the root-lesion nematodes Pratylenchus thornei and P. neglectus. Lines were grown in replicated experiments in pasteurised soil inoculated with a pure culture of either P. thornei or P. neglectus and the population density of the nematodes in the soil plus roots after 16 weeks growth was used as a measure of resistance. Combined statistical analyses of experiments (nine for P. thornei and four for P. neglectus) were conducted and genotypes were assessed using best linear unbiased predictions. Australian and international chickpea cultivars possessed a similar range of susceptibilities through to partial resistance. Wild relatives from both the primary (C. reticulatum and C. echinospermum) and secondary (C. bijugum) gene pools of chickpea were generally more resistant than commercial chickpea cultivars to either P. thornei or P. neglectus or both. Wild relatives of chickpea have probably evolved to have resistance to endemic root-lesion nematodes whereas modern chickpea cultivars constitute a narrower gene pool with respect to nematode resistance. Resistant accessions of C. reticulatum and C. echinospermum were crossed and topcrossed with desi chickpea cultivars and resistant F4 lines were obtained. Development of commercial cultivars with the high levels of resistance to P. thornei and P. neglectus in these hybrids will be most valuable for areas of the Australian grain region and other parts of the world where alternating chickpea and wheat crops are the preferred rotation.






Similar content being viewed by others
References
Abbo S, Berger J, Turner NC (2003) Evolution of cultivated chickpea: four bottlenecks limit diversity and constrain adaptation. Funct Plant Biol 30:1081–1087
Berger J, Abbo S, Turner NC (2003) Ecogeography of annual wild Cicer species: the poor state of the world collection. Crop Sci 43:1076–1090
Berger JD, Buck R, Henzell JM, Turner NC (2005) Evolution in the genus Cicer—vernalisation response and low temperature pod set in chickpea (C. arietinum L.) and its annual wild relatives. Aust J Agric Res 56:1191–1200
Berry DA (1987) Logarithmic transformations in ANOVA. Biometrics 43:439–456
Bretag TW, MacLeod WJ, Kimber RBE, Moore KJ, Knights EJC, Davidson JA (2008) Management of Ascochyta blight in chickpeas in Australia. Australas Plant Pathol 37:486–497
Castillo P, Gomez-Barcina J-D (1996) Plant parasitic nematodes associated with chickpea in southern Spain and effect of soil temperature on reproduction of Pratylenchus thornei. Nematologica 42:211–219
Castillo P, Vovlas N (2007) Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, biology, pathogenicity and management. Nematology Monographs and Perspectives 6 (Series Editors DJ Hunt, RN Perry), 529 pp. Leden-Boston, Brill
Castillo P, Jiménez-Díaz Gomez-Barcina A, Vovlas N (1995) Parasitism of the root-lesion nematode Pratylenchus thornei on chickpea. Plant Pathol 44:728–733
Castillo P, Vovlas N, Jiménez-Díaz RM (1998) Pathogenicity and histopathology of Pratylenchus thornei populations on selected chickpea genotypes. Plant Pathol 47:370–376
Collard BCY, Ades PK, Pang ECK, Brouwer JB, Taylor PWJ (2001) Prospecting for sources of resistance to Ascochyta blight in wild Cicer species. Australas Plant Pathol 30:271–276
Cook R, Evans K (1987) Resistance and tolerance. In: Brown RH, Kerry BR (eds) Principles and practice of nematode control in crops. Academic Press, New York, pp 179–231
Cox HW, Kelly RM, Strong WM (2010) Pulse crops in rotation with cereals can be a profitable alternative to nitrogen fertiliser in central Queensland. Crop and Pasture Science 61:752–762
Croser JS, Ahmad F, Clarke HJ, Siddique KHM (2003) Utilisation of wild Cicer in chickpea improvement—progress, constraints and prospects. Aust J Agric Res 54:429–444
Dalal RC, Strong WM, Weston EJ, Cooper JE, Wildermuth GB, Lehane KJ, King AJ, Holmes CJ (1998) Sustaining productivity of a Vertisol at Warra, Queensalnd with fertilisers, no-tillage or legumes. 5. Wheat yields, nitrogen benefits and water-use efficiency of chickpea-wheat rotation. Aust J Exp Agric 38:489–501
Di Vito M, Greco N, Saxena MC (1992) Pathogenicity of Pratylenchus thornei on chickpea in Syria. Nematologia mediterranea 20:71–73
Di Vito M, Zaccheo G, Catalano F (1995) Response of chickpea lines to Meloidogyne artiellia and Pratylenchus thornei. Supplement Nematologia mediterranea 23:81–83
Di Vito M, Greco N, Singh KB, Saxena MC (1996) Sources of resistance to cyst nematode in cultivated and wild Cicer species. Genet Resour Crop Evol 43:103–107
Dropkin VH (1980) Introduction to plant nematology. Wiley, New York
FAOSTAT (2011) http://faostat.fao.org/site/567/default.aspx#ancor
Felton WL, Marcellos H, Alston C, Martin RJ, Backhouse D, Burgess LW, Herridge DF (1998) Chickpea in wheat-based cropping systems of northern New South Wales. II. Influence on biomass, grain yield, and crown rot in the following wheat crop. Aust J Agric Res 49:401–407
Fortuner R (1977) Pratylenchus thornei. C.I.H. Description of Plant-parasitic Nematodes Set 7, No. 93. (Commonwealth Institute of Helminthology: St Albans, UK).
Gilmour AR, Cullis BR, Welham SJ, Thompson R (1999) ASREML Reference Manual. Biometric Bulletin No. 3. (NSW Agriculture: Orange, NSW, Australia).
Greco N (1987) Nematodes and their control in chickpea. In: Saxena MC, Singh KB (ed) The chickpea’. pp. 271–281. (CAB International: Wallingford, UK; The International Center for Agricultural Research in the Dry Areas: Syria).
Greco N, Di Vito M, Reddy MV, Saxena MC (1984) A preliminary report of survey of plant parasitic nematodes of leguminous crops in Syria. Nematologica mediterranea 12:87–93
Greco N, Di Vito M, Saxena MC, Reddy MV (1988) Investigation on the root lesion nematode, Pratylenchus thornei, in Syria. Nematologica mediterranea 16:101–105
Herridge DF, Marcellos H, Felton WL, Turner GL, Peoples MB (1995) Chickpea increases soil-N fertility in cereal systems through nitrate sparing and N2–fixation. Soil Biol Biochem 27:545–551
Hollaway GJ, Vanstone VA, Nobbs J, Smith JG, Brown JS (2008) Pathogenic nematodes of cereal crops in south-west Victoria, Australia. Australas Plant Pathol 37:505–510
Isbell RF (1996) The Australian soil classification. Revised edition. Melbourne, CSIRO Publishing
Kelly AM, Smith AB, Eccleston JA, Cullis BR (2007) The accuracy of varietal selection using factor analytic models for multi-environment plant breeding trials. Crop Sci 47:1063–1070
Knights EJ, Brinsmead RB, Fordyce M, Wood JA, Kelly A, Harden S (2002). Use of the wild relative Cicer echinospermum in chickpea improvement. In: McComb JA (ed) Plant Breeding for the 11th Millenium. Proceedings of the 12th Australasian Plant Breeding Conference, Perth, Western Australia, 15–20 September 2002, 93–111
Knights EJ, Southwell RJ, Schwinghamer MW, Harden S (2008) Resistance to Phytophthora medicaginis Hansen and Maxwell in wild Cicer species and its use in breeding root rot resistant chickpea (Cicer arietinum L.). Aust J Agric Res 59:383–387
Muehlbauer FJ, Kaiser WJ, Simon CJ (1994) Potential for wild species in cool season food legume breeding. Euphytica 73:109–114
Pande S, Galloway J, Gaur PM, Siddique KNM, Tripathi HS, Taylor P, MacLeod MWJ, Basandrai AK, Bakr A, Joshi S, Krishna Kishore G, Isenegger DA, Narayana Rao J, Sharma M (2006) Botrytis grey mould of chickpea: a review of biology, epidemiology, and disease management. Aust J Agric Res 57:1137–1150
Pande S, Sharma M, Gaur PM, Tripathi S, Kaur L, Basandrai A, Khan T, Gowda CLL, Siddique KHM (2011) Development of screening techniques and identification of new sources of resistance to Ascochyta blight disease of chickpea. Australas Plant Pathol 40:149–156
Patterson HD, Thompson R (1971) Recovery of inter-block information when block sizes are unequal. Biometrika 58:545–554
Payne RW, Harding SA, Murray DA, Soutar DM, Baird DB, Welham SJ, Kane AF, Gilmour AR, Thompson R, Webster R, Tunnicliffe Wilson G (2004) GENSTAT for Windows, 8th edn. VSN International, Oxford
Piepho H-P (1998) Empirical best linear unbiased prediction in cultivar trials using factor-analytic variance-covariance structures. Theor Appl Genet 97:195–201
Proctor JR, Marks CF (1974) The determination of normalising transformations for nematode count data from soil samples and of efficient sampling schemes. Nematologica 20:395–406
Redden RL, Berger JD (2007) History and origin of chickpea. In: Yadav SS, Redden R, Chen W, Sharma B (eds) Chickpea breeding and mangement. CABI, Wallingford, pp 1–13
Reen RA, Thompson JP (2009) A comparative study of methods for screening chickpea and wheat for resistance to root-lesion nematode Pratylenchus thornei. In Plant Health Management an Integrated Approach, 17th biennial Australasian Plant Pathology Society conference, Newcastle. (The Australasian Plant Pathology Society: Newcastle) p. 196
Riley IT, Kelly SJ (2002) Endoparasitic nematodes in cropping soils of Western Australia. Aust J Exp Agric 42:49–56
Riley IT, Wouts WM (2001) Pratylenchus and Radopholus species in agricultural soils and native vegetation in southern Australia. Trans R Soc S Aust 125:147–153
Roberts PA (2002) Concepts and consequences of resistance. In: Starr JL, Cook R, Bridge J (eds) Plant resistance to parasitic nematodes. CABI Publishing, Wallingford, pp 23–41
Robinson GK (1991) That BLUP is a good thing: the estimation of random effects. Stat Sci 6:15–51
Sheedy JG, Thompson JP (2009) Resistance to the root-lesion nematode Pratylenchus thornei of Iranian landrace wheat. Australas Plant Pathol 38:478–489
Sikora RA, Greco N (1990) Nematode parasites of food legumes. Chapter 6. In: Luc M, Sikora RA, Bridge J (eds) Plant parasitic nematodes in subtropical and tropical agriculture, 181–235.CAB International
Singh KB, Ocampo B (1997) Exploitation of wild Cicer species for yield improvement in chickpea. Theor Appl Genet 95:418–423
Singh R, Sharma P, Varshney RK, Sharma SK, Singh NK (2008) Chickpea improvement: role of wild species and genetic markers. Biotechnol Genet Eng Rev 25:267–314
Smith AB, Cullis BR, Thompson R (2001) Analysing variety by environment data using multiplicative mixed models and adjustments for spatial field trend. Biometrics 57:1138–1147
Taylor SP, Hollaway GJ, Hunt CH (2000) Effect of field crops on population densities of Pratylenchus neglectus and P. thornei in southeastern Australia; Part 1 P. neglectus. J Nematol 32:591–599
Thompson JP (1990a) Treatments to eliminate root-lesion nematode (Pratylenchus thornei Sher and Allen) from a vertisol. Nematologica 36:123–127
Thompson JP (1990b) Soil sterilization methods to show VA-mycorrhizae aid P and Zn nutrition of wheat in vertisols. Soil Biol Biochem 22:229–240
Thompson CH, Beckmann GG (1959) Soils and land use in the Toowoomba Area, Darling Downs, Queensland. CSIRO Division of Soils: Melbourne. Soils and Land Use Series 28.
Thompson JP, Haak MI (1997) Resistance to root-lesion nematode (Pratylenchus thornei) in Aegilops tauschii Coss., the D-genome donor to wheat. Aust J Agric Res 48:553–559
Thompson JP, Brennan PS, Clewett TG, Sheedy JG, Seymour NP (1999) Progress in breeding wheat for tolerance and resistance to root-lesion nematode (Pratylenchus thornei). Australas Plant Pathol 28:45–52
Thompson JP, Greco N, Eastwood R, Sharma SB, Scurrah M (2000) Integrated control of nematodes of cool-season food legumes. pp 491–506. In: Knight R (ed) Linking Research and Marketing Opportunities for Pulses in the 21st Century. Proceedings Third International Food Legumes Research Conference, Kluwer Academic Publishers, Dordrecht
Thompson JP, Owen KJ, Stirling GR, Bell MJ (2008) Root-lesion nematodes (Pratylenchus thornei and P. neglectus): a review of recent progress in managing a significant pest of grain crops in northern Australia. Australas Plant Pathol 37:235–242
Thompson JP, Clewett TG, Sheedy JG, Reen RA, O’Reilly MM (2010) Occurrence of root-lesion nematodes (Pratylenchus thornei and P. neglectus) and stunt nematode (Merlinius brevidens) in the northern grain region of Australia. Australas Plant Pathol 39:254–264
Toker C (2009) A note on the evolution of kabuli chickpeas as shown by induced mutations in Cicer reticulatum Ladizinsky. Genet Resour Crop Evol 56:7–12
Trudgill DL (1992) Resistance to and tolerance of plant-parasitic nematodes in plants. Annu Rev Phytopathol 29:167–92
Vanstone VA, Hollaway GJ, Stirling GR (2008) Managing nematode pests in the southern and western regions of the Australian cereal industry: continuing progress in a challenging environment. Australas Plant Pathol 37:220–234
Whitehead AG, Hemming JR (1965) A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann Appl Biol 55:25–38
Yadav SS, Kumaat J, Yadav SK, Singh S, Yadav VS, Turner NC, Redden R (2006) Evaluation of Helicoverpa and drought resistance in desi and kabuli chickpea. Plant Genetic Resources: Characterization and Utilization 4:198–203
Acknowledgements
This work was financially supported by the Grains Research and Development Corporation (GRDC) as part of the Australian Co-ordinated Chickpea Improvement Program (ACCIP). The following members of ACCIP kindly provided chickpea seed and/or comment on results: R. Brinsmead, M. English, J. Brouwer, C. Pittock, K. Hobson, T. Khan, W. Hawthorne, K. Siddique and T. Bretag. We also thank A. McIntyre, former Curator of the Australian Temperate Field Crops Collection, Horsham, Vic., for provision of seed of wild relatives of chickpea, and V. Vanstone for the culture of P. neglectus.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 147 kb)
Rights and permissions
About this article
Cite this article
Thompson, J.P., Reen, R.A., Clewett, T.G. et al. Hybridisation of Australian chickpea cultivars with wild Cicer spp. increases resistance to root-lesion nematodes (Pratylenchus thornei and P. neglectus). Australasian Plant Pathol. 40, 601–611 (2011). https://doi.org/10.1007/s13313-011-0089-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-011-0089-z