Abstract
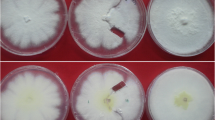
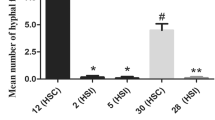
In many heterothallic fungal pathogens, mating types are found to be associated with variation in virulence and some other ecological traits. Sclerotinia trifoliorum is unique from other Sclerotinia species in that it is heterothallic with two mating types. The mating type gene has pleotropic effect on ascospore size; large ascospore isolates are phenotypically homothallic (L-type), and small ascospore isolates are heterothallic (S-type). The possible association of variation in virulence with the two mating types and hence the ascospore size in S. trifoliorum is investigated using isolates collected from naturally infected chickpea plants and isolates generated from controlled crosses. Chi-square tests showed that 57 field isolates collected from crown lesions (infection initiated by mycelium) had a 1:1 distribution of L-type (29 isolates) and S-type (28 isolates), whereas 14 isolates from stem lesions (infection initiated by ascospores) had a distribution of 10 L-type isolates and 4 S-type isolates not significantly different from 1:1. Greenhouse tests using mycelial plugs as inoculum of field and laboratory-derived isolates did not show significant difference between the two mating types in causing stem rot of chickpea. The sample size of ascospore-initiated infection was small, and the controlled pathogenicity assays in the greenhouse only tested mycelial infection. Thus, whether the two types of ascospores have equal capability of infecting chickpea remains to be further investigated. Strong evidence of both field and greenhouse data showed that mycelia of both mating types of S. trifoliorum were equally capable of infecting chickpea.


Similar content being viewed by others
References
Alvarez-Perez S, Blanco P, Garcia ME (2010) Mating type and invasiveness are significantly associated in Aspergillus fumigatus. Med Mycol 48:273–277
Butler G, Kenny C, Fagan A, Kurischko C, Gaillardin C, Wolfe KH (2004) Evolution of the MAT locus and its Ho endonuclease in yeast species. PNAS 101:1632–1637
Doerder FP, Gates MA, Eberhardt FP, Arslanyolu M (1995) High frequency of sex and equal frequencies of mating types in natural populations of the ciliate Tetrahymena thermophila. Proc Natl Acad Sci U S A 92:8715–8718
Kohn LM (1979) Delimitation of the economically important plant pathogenic Sclerotinia species. Phytopathology 69:881–886
Kosaka T (1991) Life-cycle of Paramecium bursaria syngen -1 in nature. J Protozool 38:140–148
Kwon-Chung KJ, Edman JC, Wickes BL (1992) Genetic association of mating types and virulence in Cryptococcus neoformans. Infect Immun 60:602–605
Njambere EN, Chen W, Frate C, Wu B-M, Temple SR, Muehlbauer FJ (2008) Stem and crown rot of chickpea in California caused by Sclerotinia trifoliorum. Plant Dis 92:917–922
Porter LD, Hoheisel G, Coffman VA (2009) Resistance of peas to Sclerotinia sclerotiorum in the Pisum core collection. Plant Pathol 58:52–60
Powers KS, Steadman JR, Higgins BS, Powers TO (2001) Intraspecific variation within North American Sclerotinia trifoliorum isolates characterized by nuclear small subunit rDNA introns. In: Young CS, Hughes KJD (eds) Proceedings of Sclerotinia 2001—the XI International Sclerotinia Workshop, York 8th–12th July 2001, York, England: Central Science Laboratory, York, England, p 95–96
Turgeon BG (1998) Application of mating type gene technology to problems in fungal biology. Annu Rev Phytopathol 36:115–137
Uhm JY, Fujii J (1983a) Ascospore dimorphism in Sclerotinia trifoliorum and cultural characters of strains from different-sized spores. Phytopathology 73:565–569
Uhm JY, Fujii J (1983b) Heterothallism and mating type mutation in Sclerotinia trifoliorum. Phytopathology 73:569–572
Wahl R, Zahiri A, Kämper J (2010) The Ustilago maydis b mating type locus controls hyphal proliferation and expression of secreted virulence factors in planta. Mol Microbiol 75:208–220
Willetts HJ, Wong JA-L (1980) The biology of Sclerotinia sclerotiorum, Sclerotinia trifoliorum, and Sclerotinia minor with emphasis on specific nomenclature. Bot Rev 46:101–165
Zhan J, Kema GHJ, Waalwijk C, McDonald BA (2002) Distribution of mating type alleles in the wheat pathogen Mycosphaerella graminicola over spatial scales from lesions to continents. Fungal Genet Biol 36:128–136
Zhan J, Torriani SFF, McDonald BA (2007) Significant difference in pathogenicity between MAT1-1 and MAT1-2 isolates in the wheat pathogen Mycosphaerella graminicola. Fungal Genet Biol 44:339–346
Acknowledgements
This study was funded in part by the USDA Agricultural Research Service National Sclerotinia Initiative.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Njambere, E.N., Chen, W., Frate, C. et al. Ascospore dimorphism-associated mating types of Sclerotinia trifoliorum equally capable of inducing mycelial infection on chickpea plants. Australasian Plant Pathol. 40, 648–655 (2011). https://doi.org/10.1007/s13313-011-0069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-011-0069-3