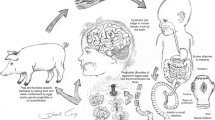
Abstract
Justification
Neurocysticercosis (NCC) is a significant problem in India and other developing countries; however, several aspects of this disease have no clear, practical guidelines. There is a need for pragmatic guidelines, summarizing the available evidence, and filling in the gaps in evidence with expert advice to manage children with neurocysticercosis.
Process
An expert group (16 members) and a writing group (8 members) was constituted, consisting of members with varied expertise. It included pediatric neurologists (18), neurologist (1), Neuroradiologists (4), and a parasitologist (1). The writing group divided the six topics and reviewed the literature on the topics individually to determine the clinical questions for which no clear guidance was available from the literature. The experts were then contacted and opinions were obtained online. The Delphi consensus method was adopted to arrive at a general consensus regarding various questions, with both the experts and the writing group members contributing. The final guidelines were then drafted by the writing group.
Recommendations
Diagnosis of NCC should be based on clinical history and neuroimaging. Contrast-enhanced magnetic resonance imaging of the brain is the modality of choice. For single enhancing lesion, albendazole therapy for 10–14 days is recommended, and it should be combined with praziquantel for 10–14 days for more than one ring-enhancing lesions. For persistent lesion, the same dose and duration of albendazole or concurrent administration of albendazole and praziquantel should be given. Pulse intravenous steroids should be used to reduce the acute symptomatic edema in children with cysticercal encephalitis. Carbamazepine or oxcarbazepine are best suited for seizure prophylaxis for those who present with seizures; phenytoin and levetiracetam are the other alternatives. In the case of NCC presenting with symptoms other than seizures, there appears to be no role for routine anti-seizure medication prophylaxis. For a single ring-enhancing lesion, six months of anti-seizure medication is sufficient if the lesion resolves on follow-up. Those with persistent lesions, calcification, or multiple lesions, require a longer treatment duration of at least 24 months.
Similar content being viewed by others
References
Coyle CM, Mahanty S, Zunt JR, et al. Neurocysticercosis: Neglected but not forgotten. PLoS Negl Trop Dis. 2012;6:e1500.
Baird RA, Wiebe S, Zunt JR, et al. Evidence-based Guideline: Treatment of Parenchymal Neurocysticercosis: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:1424–29.
Murthy JM, Subba Reddy YV. Prognosis of epilepsy associated with single CT enhancing lesion: a long term follow up study. J Neurol Sci. 1998;159:151–55.
Garcia HH, Gonzalez AE, Gilman RH. Taenia solium cysticercosis and its impact in neurological disease. Clin Microbiol Rev. 2020;33:e00085–19.
Rajshekhar V, Raghava MV, Prabhakaran V, et al. Active epilepsy as an index of burden of neurocysticercosis in Vellore district, India. Neurology. 2006;67:2135–39.
Khurana S, Aggarwal A, Malla N. Prevalence of anti-cysticercus antibodies in slum, rural and urban populations in and around Union territory, Chandigarh. Indian J Pathol Microbiol. 2006;49:51–53.
Prasad KN, Prasad A, Gupta RK, et al. Prevalence and associated risk factors of Taenia solium taeniasis in a rural pig farming community of north India. Trans R Soc Trop Med Hyg. 2007;101:1241–47.
Kumar A, Mandal A, Sinha S, et al. Prevalence, response to cysticidal therapy, and risk factors for persistent seizure in Indian children with neurocysticercosis. Int J Pediatr. 2017;2017: 8983958.
Chandy MJ, Rajshekhar V, Prakash S, et al. Cysticercosis causing single, small CT lesions in Indian patients with seizures. Lancet. 1989;1:390–91.
White AC, Coyle CM, Rajshekhar V, et al. Diagnosis and Treatment of neurocysticercosis: 2017 Clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2018;66:e49–75.
van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: Diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22:S37–62.
Gripper LB, Welburn SC. Neurocysticercosis infection and disease-A review. Acta Trop. 2017;166:218–24.
Sorvillo FJ, Waterman SH, Richards FO, Schantz PM. Cysticercosis surveillance: locally acquired and travel-related infections and detection of intestinal tapeworm carriers in Los Angeles County. Am J Trop Med Hyg. 1992;47:365–71.
Zammarchi L, Strohmeyer M, Bartalesi F, et al. Epidemiology and management of cysticercosis and Taenia solium taeniasis in Europe, systematic review 1990–2011. PloS One. 2013;8: e69537.
Proaño-Narvaez JV, Meza-Lucas A, Mata-Ruiz O, et al. Laboratory diagnosis of human neurocysticercosis: Double-blind comparison of enzyme-linked immunosorbent assay and electro-immunotransfer blot assay. J Clin Microbiol. 2002;40:2115–18.
Carod J-F, Randrianarison M, Razafimahefa J, et al. Evaluation of the performance of 5 commercialized enzyme immunoassays for the detection of Taenia solium antibodies and for the diagnosis of neurocysticercosis. Diagn Microbiol Infect Dis. 2012;72:85–89.
Del Brutto OH, Nash TE, White AC, et al. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci. 2017;372:202–10.
Singhi P, Suthar R. Neurocysticercosis. Indian J Pediatr. 2015;82:166–71.
Pretell EJ, Martinot C, Garcia HH, et al. Differential diagnosis between cerebral tuberculosis and neurocysticercosis by magnetic resonance spectroscopy. J Comput Assist Tomogr. 2005;29:112–14.
Gupta RK, Jobanputra KJ, Yadav A. MR spectroscopy in brain infections. Neuroimaging Clin N Am. 2013;23:475–98.
Matthaiou DK, Panos G, Adamidi ES, Falagas ME. Albendazole versus praziquantel in the treatment of neurocysticercosis: A meta-analysis of comparative trials. PLoS Negl Trop Dis. 2008;2:e194.
Sotelo J, del Brutto OH, Penagos P, et al. Comparison of therapeutic regimen of anticysticercal drugs for parenchymal brain cysticercosis. J Neurol. 1990;237:69–72.
Romo ML, Carpio A, Kelvin EA. Routine drug and food interactions during antihelminthic treatment of neurocysticercosis: A reason for the variable efficacy of albendazole and praziquantel? J Clin Pharmacol. 2014;54:361–7.
Garcia HH, Gilman RH, Horton J, et al. Albendazole therapy for neurocysticercosis: A prospective double-blind trial comparing 7 versus 14 days of treatment. Cysticercosis Working Group in Peru. Neurology. 1997;48:1421–27.
Kaur P, Dhiman P, Dhawan N, et al. Comparison of 1 week versus 4 weeks of albendazole therapy in single small enhancing computed tomography lesion. Neurol India. 2010;58:560–64.
Sotelo J, Penagos P, Escobedo F, Del Brutto OH. Short course of albendazole therapy for neurocysticercosis. Arch Neurol. 1988;45:1130–33.
Singhi P, Dayal D, Khandelwal N. One week versus four weeks of albendazole therapy for neurocysticercosis in children: A randomized, placebo-controlled double blind trial. Pediatr Infect Dis J. 2003;22:268–72.
Kaur S, Singhi P, Singhi S, Khandelwal N. Combination therapy with albendazole and praziquantel versus albendazole alone in children with seizures and single lesion neurocysticercosis: A randomized, placebo-controlled double blind trial. Pediatr Infect Dis J. 2009;28:403–6.
Garcia HH, Lescano AG, Lanchote VL, et al. Pharmacokinetics of combined treatment with praziquantel and albendazole in neurocysticercosis. Br J Clin Pharmacol. 2011;72:77–84.
Garcia HH, Lescano AG, Gonzales I, et al. Cysticidal efficacy of combined treatment with praziquantel and albendazole for parenchymal brain cysticercosis. Clin Infect Dis 2016;62:1375–79.
Garcia HH, Gonzales I, Lescano AG, et al. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: A double-blind, randomised controlled trial. Lancet Infect Dis. 2014;14:687–95.
Rajshekhar V. Incidence and significance of adverse effects of albendazole therapy in patients with a persistent solitary cysticercus granuloma. Acta Neurol Scand. 1998;98:121–23.
Carpio A, Hauser WA. Prognosis for seizure recurrence in patients with newly diagnosed neurocysticercosis. Neurology. 2002;59:1730–34.
Singhi P, Saini AG. Pediatric neurocysticercosis: current challenges and future prospects. Pediatric Health Med Ther. 2016; 7:5–16.
Qavi A, Garg RK, Malhotra HS, et al. Disseminated cysticercosis: clinical spectrum, Toll-like receptor-4 gene polymorphisms and role of albendazole: A prospective follow-up of 60 cases with a review of 56 published cases. Medicine (Baltimore). 2016;95:e4882.
Amaral L, Maschietto M, Maschietto R, et al. Ununsual manifestations of neurocysticercosis in MR imaging: analysis of 172 cases. Arq Neuropsiquiatr. 2003;61:533–41.
Marcin Sierra M, Arroyo M, Cadena Torres M, et al. Extraparenchymal neurocysticercosis: Demographic, clinico-radiological, and inflammatory features. PLoS Negl Trop Dis. 2017;11:e0005646.
Gupta RK, Awasthi R, Rathore RKS, et al. Understanding epileptogenesis in calcified neurocysticercosis with perfusion MRI. Neurology. 2012;78:618–25.
Alarcón F, Escalante L, Dueñas G, et al. Neurocysticercosis. Short course of treatment with albendazole. Arch Neurol. 1989;46:1231–36.
Zhao BC, Jiang HY, Ma WY, et al. Albendazole and corticosteroids for the treatment of solitary cysticercus granuloma: A network meta-analysis. PLoS Negl Trop Dis. 2016;10:e0004418.
Mall RK, Agarwal A, Garg RK, et al. Short course of prednisolone in Indian patients with solitary cysticercus granuloma and new-onset seizures. Epilepsia. 2003;44:1397–401.
Cuello-García CA, Roldán-Benítez YM, Pérez-Gaxiola G. Corticosteroids for neurocysticercosis: A systematic review and meta-analysis of randomized controlled trials. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2013;17:e583–92.
Otte WM, Singla M, Sander JW, Singh G. Drug therapy for solitary cysticercus granuloma. Neurology. 2013;80:152–62.
Modak A, Suthar R, Sharawat IK, et al. An ambispective cohort study to assess seizure recurrences in children with calcified parenchymal neurocysticercosis. Am J Trop Med Hyg. 2019;101: 812–20.
Nash TE, Ware JM, Mahanty S. Natural history of patients with perilesional edema around taenia solium calcified granulomas. J Infect Dis. 2017;215:1141–47.
Nash TE, Pretell EJ, Lescano AG, et al. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: A prospective cohort and nested case-control study. Lancet Neurol. 2008;7:1099–105.
Kaushal S, Rani A, Chopra SC, Singh G. Safety and efficacy of clobazam versus phenytoin-sodium in the antiepileptic drug treatment of solitary cysticercus granulomas. Neurol India. 2006;54:157–60.
de Souza A, Nalini A, Kovoor JME, et al. Perilesional gliosis around solitary cerebral parenchymal cysticerci and long-term seizure outcome: A prospective study using serial magnetization transfer imaging. Epilepsia. 2011;52:1918–927.
Leite JP, Terra-Bustamante VC, Fernandes RM, et al. Calcified neurocysticercotic lesions and postsurgery seizure control in temporal lobe epilepsy. Neurology. 2000;55:1485–91.
Rathore C, Thomas B, Kesavadas C, Radhakrishnan K. Calcified neurocysticercosis lesions and hippocampal sclerosis: Potential dual pathology? Epilepsia. 2012;53:e60–62.
Rathore C, Thomas B, Kesavadas C, et al. Calcified neuro-cysticercosis lesions and antiepileptic drug-resistant epilepsy: A surgically remediable syndrome? Epilepsia. 2013;54:1815–22.
Singla M, Singh P, Kaushal S, et al. Hippocampal sclerosis in association with neurocysticercosis. Epileptic Disord. 2007;9: 292–299.
Bianchin MM, Velasco TR, Wichert-Ana L, et al. How frequent is the association of neurocysticercosis and mesial temporal lobe epilepsy with hippocampal sclerosis? Epilepsia. 2010; 51: 2359–60.
Sharma M, Singh T, Mathew A. Antiepileptic drugs for seizure control in people with neurocysticercosis. Cochrane Database Syst Rev. 2015;10:CD009027.
Singhi P, Ray M, Singhi S, Khandelwal N. Clinical spectrum of 500 children with neurocysticercosis and response to albendazole therapy. J Child Neurol. 2000;15:207–13.
Verma A, Misra S. Outcome of short-term antiepileptic treatment in patients with solitary cerebral cysticercus granuloma. Acta Neurol Scand. 2006;113:174–77.
Thussu A, Arora A, Prabhakar S, Lal V, Sawhney IMS. Acute symptomatic seizures due to single CT lesions: How long to treat with antiepileptic drugs? Neurol India. 2002;50:141–44.
Gupta M, Agarwal P, Khwaja GA, et al. Randomized prospective study of outcome of short term antiepileptic treatment in small single enhancing CT lesion in brain. Neurol India. 2002;50:145–47.
Rajshekhar V, Jeyaseelan L. Seizure outcome in patients with a solitary cerebral cysticercus granuloma. Neurology. 2004; 62:2236–40.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
List of group members provided as Annexure.
Note
Additional material related to this study is available with the online version at www.indianpediatrics.net
Contributors
NS and PS: conceptualized the idea; NS, RAK, MK, LK, AK, GRP, IKS, PS: constituted the writing committee and drafted the manuscript; NS: devised and conducted Delphi process. All authors approved the final version of manuscript, and are accountable for all aspects of the manuscript.
Funding
None
Competing interest
None stated.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Sankhyan, N., Kadwa, R.A., Kamate, M. et al. Management of Neurocysticercosis in Children: Association of Child Neurology Consensus Guidelines. Indian Pediatr 58, 871–880 (2021). https://doi.org/10.1007/s13312-021-2311-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-021-2311-6