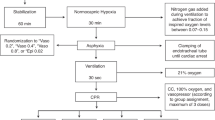
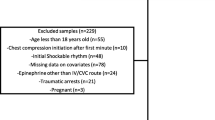
Abstract
Objective
To compare the efficacy of epinephrine plus vasopressin vs epinephrine plus placebo in the pediatric intensive care unit (PICU) cardiopulmonary resuscitation (CPR).
Design
Randomized, double-blind controlled clinical trial.
Setting
PICU in a tertiary care institute from February, 2019 to May, 2020.
Participants
Children aged one month to 13 years who required CPR during PICU stay. Patients in whom vascular access was not available or return of spontaneous circulation (ROSC) was achieved by defibrillation without epinephrine were excluded.
Intervention
Patients were randomized to receive vasopressin 0.1 mL per kg (=0.8 unit per kg) or placebo (0.1 mL per kg normal saline) in addition to epinephrine (1:10000) 0.1 mL per kg. The drugs were given as bolus doses every three minutes until the ROSC or up to a maximum of five doses, whichever was earlier.
Outcome Measure
The primary outcome was the proportion of patients who achieved ROSC. The secondary outcomes were survival rate and functional status (at 24-hour, PICU, hospital, and 90-day post-discharge), need for organ supports, length of stay (PICU and hospital), and adverse effect(s) of the study drugs.
Results
90 patients (epinephrine plus vasopressin group, n=45 and epinephrine plus placebo group, n=45) were analyzed on intention-to-treat basis. There was no significant difference in the primary outcome between epinephrine plus vasopressin (n=25, 55.5%) and epinephrine plus placebo groups (n=24, 53.3%) (Relative risk 1.04, 95% CI 0.71 to 1.52). There was no significant difference in survival rate at 24-hour (n=7, 15.6% vs. n=8, 17.8%), at PICU, hospital, and 90-day post-discharge (n=1, 2.2% vs n=1, 2.2%). There was no difference in other secondary outcomes. No trial drug-related serious adverse events were observed.
Conclusion
A combination of epinephrine plus vasopressin did not improve the rate of return of spontaneous circulation in the pediatric intensive care unit cardiopulmonary resuscitation as compared with epinephrine plus placebo.
Similar content being viewed by others
References
Duncan JM, Meaney P, Simpson P, et al. Vasopressin for in-hospital pediatric cardiac arrest: results from the American Heart Association National Registry of Cardiopulmonary Resuscitation. Pediatr Crit Care Med. 2009;10: 191–5.
de Caen AR, Berg MD, Chameides L, et al. Part 12: Pediatric Advanced Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S526–42.
Panchal AR, Berg KM, Hirsch KG, et al. 2019 American Heart Association Focused Update on Advanced Cardiovascular Life Support: Use of Advanced Airways, Vasopressors, and Extracorporeal Cardiopulmonary Resuscitation During Cardiac Arrest: An Update to the American Heart Association Guidelines for Cardio-pulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2019;140:e881–e94.
Voelckel WG, Lurie KG, McKnite S, et al. Effects of epinephrine and vasopressin in a piglet model of prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med. 2002;30:957–62.
Mann K, Berg RA, Nadkarni V. Beneficial effects of vasopressin in prolonged pediatric cardiac arrest: a case series. Resuscitation. 2002;52:149–56.
Carroll TG, Dimas VV, Raymond TT. Vasopressin rescue for in-pediatric intensive care unit cardiopulmonary arrest refractory to initial epinephrine dosing: A prospective feasibility pilot trial. Pediatr Crit Care Med. 2012;13: 265–72.
Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–20.
Zaritsky A, Nadkarni V, Hazinski MF, et al. Recommended Guidelines for Uniform Reporting of Pediatric Advanced Life Support: The Pediatric Utstein Style. Ann Emerg Med. 1995;26:487–503.
Reis AG, Nadkarni V, Perondi MB, Grisi S, Berg RA. A prospective investigation into the epidemiology of inhospital pediatric cardiopulmonary resuscitation using the international Utstein reporting style. Pediatrics. 2002;109: 200–9.
Jacobs I, Nadkarni V, Bahr J, et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update and Simplification of the Utstein Templates for Resuscitation Registries: A Statement for Healthcare Professionals from a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, Inter American Heart Foundation, Resuscitation Councils of Southern Africa). Circulation. 2004;110:3385–97.
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45.
Rathore V, Bansal A, Singhi SC, Singhi P, Muralidharan J. Survival and neurological outcome following in-hospital paediatric cardiopulmonary resuscitation in North India. Paediatr Int Child Health. 2016;36:141–7.
Donoghue AJ, Abella BS, Merchant R, et al. Cardiopulmonary resuscitation for in-hospital events in the emergency department: A comparison of adult and pediatric outcomes and care processes. Resuscitation. 2015;92: 94–100.
Ridgeon EE, Bellomo R, Aberegg SK, et al. Effect sizes in ongoing randomized controlled critical care trials. Crit Care. 2017;21:132.
Skellett S, Biarent D, Nadkarni V. What works in paediatric CPR? Intensive Care Med. 2018;44:223–6.
Agrawal A, Singh VK, Varma A, Sharma R. Therapeutic applications of vasopressin in pediatric patients. Indian Pediatr. 2012;49:297–305.
Finn J, Jacobs I, Williams TA, Gates S, Perkins GD. Adrenaline and vasopressin for cardiac arrest. Cochrane Database Syst Rev. 2019;1:CD003179.
Duncan BW, Ibrahim AE, Hraska V, et al. Use of rapid-deployment extracorporeal membrane oxygenation for the resuscitation of pediatric patients with heart disease after cardiac arrest. J Thorac Cardiovasc Surg. 1998;116: 305–11.
Morris MC, Wernovsky G, Nadkarni VM. Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2004;5:440–46.
Thourani VH, Kirshbom PM, Kanter KR, et al. Venoarterial extracorporeal membrane oxygenation (VA-ECMO) in pediatric cardiac support. Ann Thorac Surg. 2006;82:138–44; discussion 144–5.
Acknowledgments
Mrs. S. Raja Deepa B.Com, MCA (JIPMER Campus, Puducherry, India) for blinded data handling, review and editing of the manuscript; Mr. Rakesh Mohindra (Punjab University, Chandigarh, India) and Mrs. Thenmozhi M (M.Sc, Ph.D., Senior Demonstrator, CMC, Vellore, India) for helping with statistical analysis and Mrs. Harpreet Kaur (Punjab University, Chandigarh, India), and Mrs. Neelima Chadha (Tulsi Das Library, PGIMER, Chandigarh, India) for helping with the medical literature search.
Author information
Authors and Affiliations
Corresponding author
Additional information
Note
The preliminary study data was presented in the 21st National Conference of IAP Intensive Care Chapter (NCPIC 2019), from 5th to 8th December, 2019, Bengaluru.
Contributors
RR: had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis; RR: Study concept and design; AS, MC, KM, RSK, AJ, RB: acquisition, analysis, or interpretation of data and drafting of the first manuscript; MC, RB, NB, SM: protocol development and revision of the manuscript; RR, SM: critical revision of the manuscript for important intellectual content; RR, NB: study supervision. RR: is the guarantor of the paper. All authors approved the final version of the manuscript. Funding: None
Competing interest
None stated.
Rights and permissions
About this article
Cite this article
Sheriff, A., Rameshkumar, R., Chidambaram, M. et al. Epinephrine Plus Vasopressin vs Epinephrine Plus Placebo in Pediatric Intensive Care Unit Cardiopulmonary Resuscitation: A Randomized Double Blind Controlled Clinical Trial. Indian Pediatr 58, 624–630 (2021). https://doi.org/10.1007/s13312-021-2256-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-021-2256-9