Abstract
Background
There is a paucity of data on use of dexmedetomidine as a sedative agent in mechanically ventilated children.
Objectives
To compare the efficacy of dexmedetomidine and midazolam for sedation in mechanically ventilated children aged 1 month — 15 years. Secondary objectives were to compare the need for top-up doses of fentanyl and paralytic agents, duration of mechanical ventilation, ICU stay and hospital stay, and adverse events.
Design
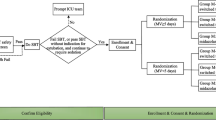
Open label, non-inferiority, randomized controlled trial.
Setting
PICU of a tertiary care teaching hospital in India.
Patients
Consecutive children aged 1 month to 15 years who were mechanically ventilated.
Intervention
Children were randomized to either dexmedetomidine or midazolam and the doses were titrated to maintain target sedation score of 4 or 5 as measured by Penn State Children Hospital Sedation algorithm.
Outcome
The percentage of time spent in level 4 or 5 of Penn State Children Hospital sedation algorithm for ventilated children.
Results
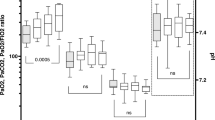
49 children were randomized (24 to ‘midazolam group’ and 25 to ‘dexmedetomidine group’). There was no difference in the percentage of time spent in the targeted sedation between the groups [midazolam 67.3% (18.8) vs. dexmedetomidine 56.3 %. (28.6); P=0.12]. The absolute difference in the percentage of time spent was −10.9% [SE (95% CI) 7.05: (−25.15 to 3.25)]. The lower end of 95% CI for the difference breached the non-inferiority limit of −20%. Number of fentanyl boluses, duration of mechanical ventilation, ICU stay, and hospital stay were similar. Four (17.4%) children in dexmedetomidine group developed persistent bradycardia.
Conclusion
Non-inferiority of dexmedetomidine compared to midazolam for sedation in children on mechanical ventilation could not be established.
Similar content being viewed by others
References
Rhoney DH, Murry KR. National survey on the use of sedatives and neuromuscular blocking agents in the pediatric intensive care unit. Pediatr Crit Care Med. 2002;3:129–13.
Panzer O, Moitra V, Sladen RN. Pharmacology of sedative-analgesic agents: Dexmedetomidine, remifentanil, ketamine, volatile anesthetics, and the role of peripheral mu antagonists. Crit Care Clin. 2009;25:451–69.
Andreolio C, Piva JP, Baldasso E, Ferlini R, Piccoli R. Prolonged infusion of dexmedetomidine in critically-ill children. Indian Pediatr. 2016;53:987–9.
Sperotto F, Mondardini MC, Vitale F, et al. Prolonged sedation in critically ill children: is dexmedetomidine a safe option for younger age? An off-label experience. Minerva Anestesiol. 2019;85;164–72.
Shutes BL, Gee SW, Sargel CL, Fink KA, Tobias JD. Dexmedetomidine as single continuous sedative during noninvasive ventilation: typical usage, hemodynamic effects, and withdrawal. Pediatr Crit Care Med. 2018;19:287–97.
Constantin JM, Momon A, Mantz J, et al. Efficacy and safety of sedation with dexmedetomidine in critical care patients: A meta-analysis of randomized controlled trials. Anaesth Crit Care Pain Med. 2016;35:7–15.
Walker J, MacCallum M, Fischer C, Kopcha R, Saylors R, McCall J. Sedation using dexmedetomidine in pediatric burn patients. J Burn Care Res. 2006;27:206–10.
Whalen LD, Di Gennaro JL, Irby GA, Yanay O, Zimmerman JJ. Long-term dexmedetomidine use and safety profile among critically ill children and neonates. Pediatr Crit Care Med. 2014;15:706–14.
Reiter PD, Pietras M, Dobyns EL. Prolonged dexmedetomidine infusions in critically ill infants and children. Indian Pediatr. 2009;46:767–73.
Benneyworth BD, Downs SM, Nitu M. Retrospective evaluation of the epidemiology and practice variation of dexmedetomidine use in invasively ventilated pediatric intensive care admissions, 2007–2013. Front Pediatr. 2015; 3:109.
Lang B, Zhang L, Zhang W, Lin Y, Fu Y, Chen S. A comparative evaluation of dexmedetomidine and midazolam in pediatric sedation: A meta analysis of randomized controlled trials with trial sequential analysis. CNS Neurosci Ther. 2020;8: 862–75.
Tobias JD, Berkenbosch JW. Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam. South Med J. 2004;97:451–56.
Prasad S, Simha P, Jagadeesh A. Comparative study between dexmedetomidine and fentanyl for sedation during mechanical ventilation in post-operative paediatric cardiac surgical patients. Indian J Anaesth. 2012;56:547–52.
Garisto C, Ricci Z, Tofani L, Benegni S, Pezzella C, Cogo P. Use of low dose dexmedetomidine in combination with opioids and midazolam in paediatric cardiac surgical patients: randomized controlled trial. Minerva Anestesiol. 2018;84:1053–62.
Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA. 2009;301:489–99.
Popernack ML, Thomas NJ, Lucking SE. Decreasing unplanned extubations: Utilization of the Penn State Children’s Hospital Sedation Algorithm. Pediatr Crit Care Med. 2004;5:58–62.
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76.
Jakob SM, Ruokonen E. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation. JAMA. 2012;307:1151–60.
Grant MJ, Schneider JB, Asaro LA, et al. Dexmedetomidine use in critically ill children with acute respiratory failure. Pediatr Crit Care Med. 2016;17:1131–41.
Holliday SF, Kane-Gill SL, Empey PE, Buckley MS, Smithburger PL. Interpatient variability in dexmedetomidine response: A survey of the literature. Sci World J. 2014;2014: 805013.
Tellor BR, Arnold HM, Micek ST, Kollef MH. Occurrence and predictors of dexmedetomidine infusion intolerance and failure. Hosp Pract. 2012;40:186–92.
Kurnik D, Muszkat M, Sofowora GG, et al. Ethnic and genetic determinants of cardiovascular response to the selective 2-adrenoceptor agonist dexmedetomidine. Hypertension. 2008;51:406–11.
Iirola T, Ihmsen H, Laitio R, et al. Population pharmacokinetics of dexmedetomidine during long-term sedation in intensive care patients. Br J Anaesth. 2012;108:460–68.
Smithburger PL, Smith RB, Kane-Gill SL, Empey PE. Patient predictors of dexmedetomidine effectiveness for sedation in intensive care units. Am J Crit Care. 2014;23:160–65.
Fujita Y, Inoue K, Sakamoto T, et al. Relationship between dexmedetomidine dose and plasma dexmedetomidine concentration in critically ill infants: a prospective observational cohort study. Korean J Anesthesiol 2017;70:426–33.
Jones GM, Murphy CV, Gerlach AT, Goodman EM, Pell LJ. High-dose dexmedetomidine for sedation in the intensive care unit: an evaluation of clinical efficacy and safety. Ann Pharmacother. 2011;45:740–7.
Yaðar S, Yavab S, Karahalil B. The role of the ADRA2A C1291G genetic polymorphism in response to dexmedetomidine on patients undergoing coronary artery surgery. Mol Biol Rep. 2011;38:3383–9.
Funding
None
Author information
Authors and Affiliations
Contributions
KMG: design of the study, patient management, data collection, data analysis, and preparation of manuscript; JS,KRG,SKK: design of the study, patient management, reviewed manuscript; RL: design of the study, patient management and reviewed manuscript.
Corresponding author
Ethics declarations
Ethics clearance: The study was approved by Institute Ethics Committee, AIIMS, New Delhi; IECPG/403, dated June 29, 2016.
Competing interest: None stated.
Rights and permissions
About this article
Cite this article
Gulla, K.M., Sankar, J., Jat, K.R. et al. Dexmedetomidine vs Midazolam for Sedation in Mechanically Ventilated Children: A Randomized Controlled Trial. Indian Pediatr 58, 117–122 (2021). https://doi.org/10.1007/s13312-021-2124-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-021-2124-7