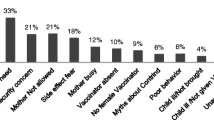
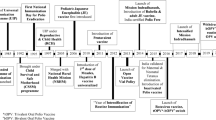
Abstract
Immunization is an established, cost-effective, preventive intervention to improve child survival. To provide protection against vaccine preventable diseases, all countries in the world have an immunization program that offers selected vaccines to the eligible beneficiaries. In India, Expanded Program of Immunization was started in 1978, and then Universal Immunization Program was launched in 1985 with six antigens. This article describes the experience with institutionalization of four state-specific vaccines by Delhi in its immunization schedule to enlarge the ambit of immunization services. It attempts to highlight the state’s perspective in terms of the implementation policy, operational strategy adopted and evolution of immunization program in the state over 16 years.
Similar content being viewed by others
References
Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008;86:140–6.
Economic Survey of Delhi 2014-15-Demographic Profile of Delhi: p.1-17. Available from:http://delhi.gov.in/wps/wcm/connect/DoIT_Planning/planning/economic+survey+of+dehli/economic+survey+of+delhi+2014+-+2015. Accessed July 16, 2016.
Census of India: Migration, Govt. of India, Ministry of Home Affairs; Available from: censusindia.gov.in/Census_And_You/migrations.asp. Accessed July 16, 2016
Dietz VJ, Lewin M, Zell E, Rodewald L. Evaluation of failure to follow vaccination recommendations as a marker for failure to follow other health recommendations. Pediatr Infect Dis J. 1997;16:1157–61.
National Family Health Survey 2, 1998-99; Factsheet Delhi: (1-9) Available from: http://rchiips.org/nfhs/data/dl/dlfctsum.pdf. Accessed July 16, 2016.
World Health Organization. Measles vaccines: WHO position paper. Wkly Epidemiol Rec. 2009;84:349–60.
WHO global status of mumps immunization and surveillance. Weekly Epidemiol Rec. 2005;80:417–24.
Gupta E, Dar L, Broor S. Seroprevalence of rubella in pregnant women in Delhi, India. Indian J Med Res. 2006;123:833–5.
Operational guidelines for the introduction of Hepatitis B vaccine in UIP of India, MoHFW, Govt. of India. 2011;3-10.
WHO Hepatitis B Fact sheet N°204; Available from: http://www.who.int/mediacentre/factsheets/fs204/en/. Accessed July 16, 2016.
Myron M. Levine. Use of Vaccines for the Prevention of Typhoid Fever. Indian Pediatr. 2003;40:1029–34. Available from: http://www.indianpediatrics.net/nov2003/nov-1029-1034.htm. Accessed July 16, 2016.
Ahmed ZA, Sunil K. Multidrug-resistant typhoid fever: a review. J Infect Dev Ctries. 2011;5:324–37.
Sinha A, Sazawal S, Kumar R, Sood S, Reddaiah VP, Singh B, et al. Typhoid fever in Children aged less than 5 years. Lancet. 1999;354:734–7.
Sharma P, Taneja D. Typhoid vaccine: A case for inclusion in national program. Indian J Public Health. 2011;55:267–71.
Subcommittee on Introduction of Hib Vaccine in Universal Immunization Program, National Technical Advisory Group on Immunization, India. NTAGI subcommittee recommendations on Haemophilus influenzae type b (Hib) vaccine introduction in India. Indian Pediatr. 2009;46:945–54.
Operational guidelines: Introduction of Hib as Pentavalent Vaccine in Universal Immunization program in India, Ministry of Health and Family Welfare, Govt. of India, New Delhi, 2011.
Das BK, Arora NK, Mathur P, Ostwal P, Mandal S, Kabra SK, et al. Nasopharyngeal carriage of Haemophilus influenzae. Indian J Pediatr. 2002;69:775–7.
Cabezas C, Echevarría C, Gómez G, Gotuzzo E. Pilot program of immunization against viral hepatitis B, integrated in the extended immunization program in Abancay (Peru). Rev Gastroenterol Peru. 1995;15:215–22.
Cui FQ, Gong XH, Chen YS. Evaluation on impact of hepatitis B vaccine integrated into routine immunization in the areas of Ministry of Health/Global Alliance for Vaccine and Immunization (GAVI) Cooperation Project P.R. China. Zhongguo Yi Miao He Mian Yi. 2009;15:289–93.
Opstelten W, Hak E, Verheij TJ, van Essen GA. Introducing a pneumococcal vaccine to an existing influenza immunization program: vaccination rates and predictors of noncompliance. Am J Med. 111:474–9.
Liu W, Clemens JD, Kari K, Xu ZY. Immunization against Japanese encephalitis in China: A policy analysis. Vaccine. 2006;24:5178–82.
Rani M, Yang B, Nesbit R. Hepatitis B control by 2012 in the WHO Western Pacific Region: rationale and implications. Bull World Health Organ. 2009;87:707–13.
Griffiths UK, Korczak VS, Ayalew D, Yigzaw A. Incremental system costs of introducing combined DTwP hepatitis B-Hib vaccine into national immunization services in Ethiopia. Vaccine. 2009;27:1426–32.
Balinska MA. Hepatitis B vaccination and French Society ten years after the suspension of the vaccination campaign: how should we raise infant immunization coverage rates? J Clin Virol. 2009;46:202–5.
Kumar D, Aggarwal AK, Kumar R. The effect of interrupted 5-day training on Integrated management of neonatal and childhood illness on the knowledge and skills of primary health care workers. Health Policy Planning. 2009;24:94–100.
Key Indicators for Delhi from NFHS-3. Available from http://rchiips.org/nfhs/pdf/Delhi.pdf. Accessed July 16, 2016.
An analysis of levels and trend in Infant and child mortality rates in India National Institute Of Public Cooperation and Child Development, New Delhi: 2014;7-42. Available from http://nipccd.nic.in/reports/imr.pdf. Accessed July 16, 2016.
Childhood Mortality and Health in India. Working Paper Series No. E/292/2008 Institute of Economic Growth, University of Delhi; Available from http://www.iegindia.org/workpap/wp292.pdf. Accessed July 16, 2016.
Valencia-Mendoza A, Bertozzi SM, Gutierrez JP, Itzle R. Cost-effectiveness of introducing a Rotavirus vaccine in developing countries: the case of Mexico. BMC Infect Dis. 2008;8:103.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raoot, A., Dewan, D.K., Dubey, A.P. et al. Introduction of new vaccines in state immunization schedule — Delhi’s experience. Indian Pediatr 54, 271–274 (2017). https://doi.org/10.1007/s13312-017-1085-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-017-1085-3