Abstract
Objective
We assessed detection of recent Japanese encephalitis virus infection using recommended strategy.
Methods
Cross-sectional community-based study conducted in 12 villages in Kushinagar, Uttar-Pradesh, India in 2012-13. Recent infection with Japanese encephalitis virus in 239 healthy children aged 1-15 year was detected using a combination of serology and molecular methods.
Results
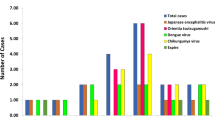
24 (10%) children showed recent infection; 2 by serology and 22 by molecular method. Symptomatic cases were estimated as 626 in Kushinagar against reported 139 in all age groups across the state.
Conclusion
Lower positivity using recommended serology suggests major gap in existing surveillance and diagnostic protocols and estimation of burden of Japanese encephalitis.
Similar content being viewed by others
References
Directorate of National Vector Borne Disease Control Program. Guidelines for Surveillance of Acute Encephalitis Syndrome (with special reference to Japanese encephalitis) [Internet]. Health (San Francisco). New Delhi; 2006. Available from: http://www.nvbdcp.gov.in/Doc/AES guidelines.pdf. Accessed August 28, 2014.
World Health Organization. WHO-recommended Standards for Surveillance of Selected Vaccine Preventable Diseases. Geneva: World Health Organization; 2008.
Hills S, Dabbagh A, Jacobson J, Marfin A, Featherstone D, Hombach J, et al. Evidence and rationale for the World Health Organization recommended standards for Japanese encephalitis surveillance. BMC Infect Dis. 2009;9:214.
Ranjan P, Gore M, Selvaraju S, Kushwaha KP, Srivastava DK, Murhekar M. Changes in acute encephalitis syndrome incidence after introduction of Japanese encephalitis vaccine in a region of India. J Infect. 2014;69:202–4.
Kakkar M, Rogawski ET, Abbas SS, Chaturvedi S, Dhole TN, Hossain SS, et al. Wishful thinking blurs interpretation of AES data in a high endemic region of India. J Infect. 2014;69:520–1.
Robinson JS, Featherstone D, Vasanthapuram R, Biggerstaff BJ, Desai A, Ramamurty N, et al. Evaluation of three commercially available Japanese encephalitis virus IgM enzyme-linked immunosorbent assays. Am J Trop Med Hyg. 2010;83:1146–55.
Gajanana A, Thenmozhi V, Samuel PP, Reuben R. A community-based study of subclinical flavivirus infections in children in an area of Tamil Nadu, India, where Japanese encephalitis is endemic. Bull World Health WHAT THIS STUDY ADDS? • Serology (IgM-MAC-ELISA) underestimates recent infection with Japanese encephalitis virus, in comparison to real time reverse transcriptase PCR. Organ.1995;73:237–44.
Panbio Diagnostics. Japanese Encephalitis-Dengue IgM Combo ELISA Test. Brisbane: Panbio Diagnostics; 2008. p. 1–6. Available from: http://alere.co.jp/products02/ panbio/japanese_encephalitis.pdf. Accessed August 28, 2014.
Saxena V, Mishra VK, Dhole TN. Evaluation of reversetranscriptase PCR as a diagnostic tool to confirm Japanese encephalitis virus infection. Trans R Soc Trop Med Hyg. 2009;103:403–6.
WHO Regional Office for South East Asia. Fourth Biregional Meeting on the Control of Japanese Encephalitis (JE). 2010.
Tiwari S, Chitti SVP, Mathur A, Saxena SK. Japanese encephalitis virus: an emerging pathogen. Am J Virol. 2012;1:1–8.
Yang KD. A model to study neurotropism and persistency of Japanese encephalitis virus infection in human neuroblastoma cells and leukocytes. J Gen Virol. 2004;85:635–42.
Sarkar A, Banik A, Pathak BK, Mukhopadhyay SK, Chatterjee S. Envelope protein gene based molecular characterization of Japanese encephalitis virus clinical isolates from West Bengal, India: A comparative approach with respect to SA14-14-2 live attenuated vaccine strain. BMC Infect Dis. 2013;13:368.
Sarkar A, Taraphdar D, Mukhopadhyay SK, Chakrabarti S, Chatterjee S. Molecular evidence for the occurrence of Japanese encephalitis virus genotype I and III infection associated with acute encephalitis in patients of West Bengal, India, 2010. Virol J. 2012;9:271.
Directorate of National Vector Borne Disease Control Program. Details of AES/JE Cases and Deaths from 2008- 2014. Available from: http://www.nvbdcp.gov.in/Doc/jeaes- cd-Aug14.pdf. Accessed August 28, 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kakkar, M., Dhole, T.N., Rogawski, E.T. et al. Public health laboratory surveillance and diagnosis of Japanese encephalitis: Time to revisit. Indian Pediatr 53, 33–35 (2016). https://doi.org/10.1007/s13312-016-0785-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-016-0785-4