Abstract
Objective
To compare the efficacy and adverse effects of aerosolized L-epinephrine vs budesonide in the treatment of post-extubation stridor.
Study design
Randomized controlled trial.
Setting
Pediatric intensive care unit (PICU) of a tertiary teaching and referral hospital.
Subjects
Sixty two patients with a stridor score ≥4 following extubation.
Intervention
Patients were randomized to receive either aerosolized L-epinephrine (n=32) or budesonide (n =30). Respiratory rate, heart rate, stridor score, blood pressure and oxygen saturation were recorded from 0 min to 24 hours.
Outcome measures
Stridor score remaining at ≥4, need for re-nebulization and re-intubation between 20 min −24 hours were primary outcome measures. Tachycardia (HR > normal for age), hypertension (BP >95th centile for age) and hypoxia (SpO2 <92% for 5 min) were secondary outcome measures.
Results
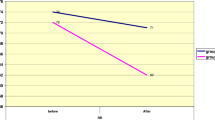
Both drugs showed a significant and comparable decline in the median (95% CI) stridor scores from baseline to 60 min [4 (4.10–4.50) to 2.00 (1.46–2.67) for budesonide vs 4 (4.12–5.00) to 2.00 (1.31–2.75) for epinephrine]. At 2 hours, the stridor scores were significantly lower in the epinephrine as compared to budesonide group [0.00 (0.69–1.81) vs 3.00(1.75–3.32); P=0.02)]. However, the proportion of patients with stridor score ≥4 at any time between 20min–24 hrs (53.3% vs 53.1%; P=0.99), need for renebulization (40 % vs 43.8 %; P=0.76) and re-intubation (20% vs 25%, P=0.638), and adverse effects were similar in both groups.
Conclusions
Both aerosolized L-epinephrine and budesonide were equally effective in their initial therapeutic response in post-extubation stridor. However, epinephrine showed a more sustained effect.
Similar content being viewed by others
References
McGovern FH, Fitz-Hugh GS, Edgemon LJ. The hazards of endotracheal intubation. Ann Otol 1971; 80: 556–564.
Anene O, Meert KL, Uy H, Simpson P, Sarnaik AP. Dexamethasone for the prevention of post extubation airway obstruction: A prospective, randomized, double-blind, placebo-controlled trial. Crit Care Med 1996; 24: 1666–1669.
Jordan WS, Graves CL, Elwyn RA. New therapy for post intubation laryngeal edema and tracheitis in children. JAMA 1970; 212: 585–588.
Goddard JE, Phillips OC, Marcy JH. Betamethasone for prophylaxis of postintubation inflammation: A double blind study. Anesth Analg 1967; 46: 348–353.
Pender JW. Endotracheal anesthesia in children: advantages and disadvantages. Anesthesiology 1954; 15: 495–506.
Nutman J, Brooks LJ, Deakins K, Baldesare K, Witte M, Reed M. Racemic versus L-epinephrine aerosol in the treatment of postextubation laryngeal edema: Results from a prospective, randomized, double-blind study. Crit Care Med 1994; 22: 1591–1594.
Waisman Y, Klein BL, Boenning DA, Young GM, Chamberlain JM, O’Donnell R, et al. Prospective randomized double-blind study comparing L-epinephrine and racemic epinephrine aerosols in the treatment of laryngotracheitis (croup). Pediatrics 1992; 89: 302–306.
Meade MO, Guyatt GH, Cook DJ, Sinuff T, Butler R. Trials of corticosteroids to prevent postextubation airway complications. Chest 2001; 120: 464S–468S.
Husby S, Agertoft L, Mortenson S, Pedersen S. Treatment of croup with nebulised steroid (budesonide): a double bind, placebo controlled study. Arch Dis Child 1993; 63: 352–355.
Godden CW, Campbell MZ, Hussey M, Cogswell JJ. Double blind placebo controlled trial of nebulised budesonide for croup. Arch Dis Child 1997; 76: 155–158.
Fitzgerald D, Mellis C, Johnson M, Allen H, Cooper P, Van Asperen P. Nebulized budesonide is as effective as nebulized adrenaline in moderately severe croup. Pediatrics 1996; 97: 722–725.
Tellez DW, Galvis AG, Storgion SA, Amer HN, Hoseyni M, Deakers TW. Dexamethasone in the prevention of postextubation stridor in children. J Pediatr 1991; 118: 289–293.
Kemper JK, Benson MS, Bishop MJ. Predictors of postextubation stridor in pediatric trauma patients. Crit Care Med 1991; 19: 352–355.
Koka BV, Andre JM, Smith RM, Jeon IS, Mackay I. Postintubation croup in children. Anesth Analg 1977; 56: 502–505.
Westley CR, Cotton EK, Brooks JG. Nebulized racemic epinephrine by IPPB for the treatment of croup. Am J Dis Child 1978; 132: 484–487.
Klassen P, Feldman E, Watters K, Sutcliffe T, Rowe C. Nebulized budesonide for children with mild-to-moderate croup. N Engl J Med 1994; 331: 285–289.
Fogel JM, Berg IJ, Gerber MA, Sherter CB. Racemic epinephrine in the treatment of croup: nebulization alone versus nebulization with intermittent positive pressure breathing. J Pediatr 1982; 25: 1028–1031.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sinha, A., Jayashree, M. & Singhi, S. Aerosolized L-epinephrine vs budesonide for post-extubation stridor: A randomized controlled trial . Indian Pediatr 47, 317–322 (2010). https://doi.org/10.1007/s13312-010-0060-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-010-0060-z