Abstract
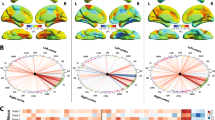
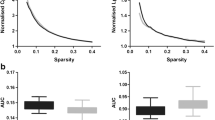
Magnetic resonance-guided focused ultrasound (MRgFUS) has brought thalamotomy back to the frontline for essential tremor (ET). As functional organization of human brain strictly follows hierarchical principles which are frequently deficient in neurological diseases, whether additional damage from MRgFUS thalamotomy induces further disruptions of ET functional scaffolds are still controversial. This study was to examine the alteration features of brain functional frameworks following MRgFUS thalamotomy in patients with ET. We retrospectively obtained preoperative (ETpre) and postoperative 6-month (ET6m) data of 30 ET patients underwent MRgFUS thalamotomy from 2018 to 2020. Their archived functional MR images were used to functional gradient comparison. Both supervised pattern learning and stepwise linear regression were conducted to associate gradient features to tremor symptoms with additional neuropathophysiological analysis. MRgFUS thalamotomy relieved 78.19% of hand tremor symptoms and induced vast global framework alteration (ET6m vs. ETpre: Cohen d = − 0.80, P < 0.001). Multiple robust alterations were identified especially in posterior cingulate cortex (\({\mathrm{ET}}_{6\mathrm{m}}\) ET6m vs. \({\mathrm{ET}}_{\mathrm{pre}}\) ETpre: Cohen d = 0.87, P = 0.048). Compared with matched health controls (HCs), its gradient distances to primary communities were significantly increased in \({\mathrm{ET}}_{\mathrm{pre}}\) ETpre patients with anomalous stepwise connectivity (P < 0.05 in ETpre vs. HCs), which were restored after MRgFUS thalamotomy. Both global and regional gradient features could be used for tremor symptom prediction and were linked to neuropathophysiological features of Parkinson disease and oxidative phosphorylation. MRgFUS thalamotomy not only suppress tremor symptoms but also rebalances atypical functional hierarchical architecture of ET patients.




Similar content being viewed by others
Abbreviations
- AHBA:
-
Allen Human Brain Atlas
- CRST:
-
Clinical Evaluation Scale for Tremor
- DA:
-
Dorsal attention
- DMN:
-
Default mode network
- ET:
-
Essential tremor
- FDA:
-
Food and Drug Administration
- FDR:
-
False discovery rate
- FP:
-
Frontoparietal
- GRETNA:
-
Graph theoretical network analysis
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- LASSO:
-
Least absolute shrinkage and selection operator
- Lim:
-
Limbic
- MNI:
-
Montreal Neurological Institute
- MRI:
-
Magnetic resonance imaging
- MRgFUS:
-
Magnetic resonance-guided focused ultrasound
- ROI:
-
Region of interest
- PLSR:
-
Partial least squares regression
- SFC:
-
Stepwise connectivity estimation
- SM:
-
Somatomotor
- VA:
-
Ventral attention
- Vim:
-
Thalamic ventral intermediate
- Vis:
-
Visual
References
Ghanouni P, Pauly KB, Elias WJ, Henderson J, Sheehan J, Monteith S, et al. Transcranial MRI-guided focused ultrasound: a review of the technologic and neurologic applications. 2015;205(1):150–9.
Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730–9.
Okun MS, Vitek JL. Lesion therapy for Parkinson’s disease and other movement disorders: update and controversies. J Mov Disord. 2004;19(4):375–89.
Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci USA. 2016;113(44):12574–9.
Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10(9):659–69.
Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res. 1982;6(1):57–77.
Huntenburg JM, Bazin PL, Margulies DS. Large-scale gradients in human cortical organization. Trends Cogn Sci. 2018;22(1):21–31.
Hong SJ, Vos de Wael R, Bethlehem RAI, Lariviere S, Paquola C, Valk SL, et al. Atypical functional connectome hierarchy in autism. Nat Commun. 2019;10(1):1022.
Bayrak Ş, Khalil AA, Villringer K, Fiebach JB, Villringer A, Margulies DS, et al. The impact of ischemic stroke on connectivity gradients. NeuroImage Clin. 2019;24:101947.
Meng Y, Yang S, Chen H, Li J, Xu Q, Zhang Q, et al. Systematically disrupted functional gradient of the cortical connectome in generalized epilepsy: initial discovery and independent sample replication. Neuroimage. 2021;230:117831.
Lambert C, Simon H, Colman J, Barrick TR. Defining thalamic nuclei and topographic connectivity gradients in vivo. Neuroimage. 2017;158:466–79.
Guell X, Schmahmann JD, Gabrieli J, Ghosh SS. Functional gradients of the cerebellum. eLife. 2018;7.
Llinás R, Ribary U, Jeanmonod D, Cancro R, Kronberg E, Schulman J, et al. Thalamocortical dysrhythmia I.: functional and imaging aspects. Thalamus & Related Systems. 2001;1(3):237–44.
Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113.
Lin J, Kang X, Xiong Y, Zhang D, Zong R, Yu X, et al. Convergent structural network and gene signatures for MRgFUS thalamotomy in patients with Parkinson’s disease. Neuroimage. 2021;243:118550.
Wang J, Wang X, Xia M, Liao X, Evans A, He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci. 2015;9:386.
Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cerebral cortex (New York, NY : 1991) 2018;28(9):3095–114.
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–45.
Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108(8):2242–63.
Horn A, Kühn AA. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 2015;107:127–35.
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–65.
Vos de Wael R, Benkarim O, Paquola C, Lariviere S, Royer J, Tavakol S, et al. BrainSpace: a toolbox for the analysis of macroscale gradients in neuroimaging and connectomics datasets. Commun Biol. 2020;3(1):103.
Hong SJ, Xu T, Nikolaidis A, Smallwood J, Margulies DS, Bernhardt B, et al. Toward a connectivity gradient-based framework for reproducible biomarker discovery. Neuroimage. 2020;223: 117322.
Langs G, Golland P, Ghosh SS. Predicting activation across individuals with resting-state functional connectivity based multi-atlas label fusion. Cham: Springer International Publishing; 2015;313–20.
Sepulcre J, Sabuncu MR, Yeo TB, Liu H, Johnson KA. Stepwise connectivity of the modal cortex reveals the multimodal organization of the human brain. J Neurosci. 2012;32(31):10649–61.
Sepulcre J. Functional streams and cortical integration in the human brain. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2014;20(5):499–508.
Markello RD, Arnatkeviciute A, Poline JB, Fulcher BD, Fornito A, Misic B. Standardizing workflows in imaging transcriptomics with the abagen toolbox. Elife. 2021;10.
Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019;47(W1):W199–205.
van Wijk BC, Stam CJ, Daffertshofer A. Comparing brain networks of different size and connectivity density using graph theory. PLoS ONE. 2010;5(10):e13701.
Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–73.
van den Heuvel MP, de Lange SC, Zalesky A, Seguin C, Yeo BTT, Schmidt R. Proportional thresholding in resting-state fMRI functional connectivity networks and consequences for patient-control connectome studies: issues and recommendations. Neuroimage. 2017;152:437–49.
Zeng Q, Guan X, Guo T,Law Yan Lun JC, Zhou C, Luo X, et al. The ventral Intermediate nucleus differently modulates subtype-related networks in Parkinson’s disease. 2019;13:202.
Schweighofer N, Doya K, Kuroda SJBRR. Cerebellar aminergic neuromodulation: towards a functional understanding. 2004;44(2–3):103–16.
Ruppert MC, Greuel A, Freigang J, Tahmasian M, Maier F, Hammes J, et al. The default mode network and cognition in Parkinson’s disease: a multimodal resting-state network approach. Hum Brain Mapp. 2021;42(8):2623–41.
Bejr-Kasem H, Pagonabarraga J, Martínez-Horta S, Sampedro F, Marín-Lahoz J, Horta-Barba A, et al. Disruption of the default mode network and its intrinsic functional connectivity underlies minor hallucinations in Parkinson’s disease. J Mov Disord. 2019;34(1):78–86.
Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38.
Rodriguez-Sabate C, Morales I, Sanchez A, Rodriguez M. The functional interaction of the brain default network with motor networks is modified by aging. Behav Brain Res. 2019;372:112048.
Yang J, Lei D, Peng J, Suo X, Pinaya WHL, Li W, et al. Disrupted brain gray matter networks in drug-naïve participants with essential tremor. Neuroradiology. 2021;63(9):1501–10.
Benito-León J, Louis ED, Romero JP, Hernández-Tamames JA, Manzanedo E, Álvarez-Linera J, et al. Altered functional connectivity in essential tremor: a resting-state fMRI study. Medicine. 2015;94(49):e1936.
Fang W, Chen H, Wang H, Zhang H, Liu M, Puneet M, et al. Multiple resting-state networks are associated with tremors and cognitive features in essential tremor. J Mov Disord. 2015;30(14):1926–36.
Peng J, Yang J, Li J, Lei D, Li N, Suo X, et al. Disrupted brain functional network topology in essential tremor patients with poor sleep quality. Front Neurosci. 2022;16:814745.
Li JY, Suo XL, Li NN, Lei D, Peng JX, Yang J, et al. Disrupted brain network topology in drug-naïve essential tremor patients with and without depression : a resting state functional magnetic resonance imaging study. Clin Neuroradiol. 2021;31(4):981–92.
Tian Q, Wintermark M, Jeffrey Elias W, Ghanouni P, Halpern CH, Henderson JM, et al. Diffusion MRI tractography for improved transcranial MRI-guided focused ultrasound thalamotomy targeting for essential tremor. NeuroImage Clin. 2018;19:572–80.
Xiong Y, Han D, He J, Zong R, Bian X, Duan C, et al. Correlation of visual area with tremor improvement after MRgFUS thalamotomy in Parkinson’s disease. J Neurosurg. 2022;136(3):681–8.
Jang C, Park H-J, Chang WS, Pae C, Chang JW. Immediate and longitudinal alterations of functional networks after thalamotomy in essential tremor. Front Neurol. 2016;7:184.
Stanziano M, Golfrè Andreasi N, Messina G, Rinaldo S, Palermo S, Verri M, et al. Resting state functional connectivity signatures of MRgFUS Vim thalamotomy in Parkinson’s disease: a preliminary study. Front Neurol. 2021;12:786734.
Koga S, Ishaque M, Jeffrey Elias W, Shah BB, Murakami A, Dickson DW. Neuropathology of Parkinson’s disease after focused ultrasound thalamotomy. NPJ Parkinson’s disease. 2022;8(1):59.
Tarakad A, Jankovic J. Essential tremor and Parkinson’s disease: exploring the relationship. Tremor Other Hyperkinet Mov (N Y). 2018;8:589.
Yoo YM, Lee CJ, Lee U, Kim YJ. Mitochondrial DNA in patients with essential tremor. Neurosci Lett. 2008;434(1):29–34.
Acknowledgements
We would also like to express our heartfelt thanks to Professors Yuesong Pan and Miao Liu for their guidance in the paper preparation.
Funding
This research was supported by National Natural Science Foundation of China 82151309, 81825012, and 81730048 to XL as well as China Postdoctoral Science Foundation 2022T150788 to JJL.
Author information
Authors and Affiliations
Contributions
Conceptualization: XL, LSP, JJL, XPK; methodology: JJL, XPK, DKZ, JYZ, XBB, DZ, XYW; investigation: DKZ, HXL, XYG; visualization: JJL, DKZ; supervision: XGY, LSP, XL, JS; writing—original draft: JJL, XPK, JS; writing—review and editing: JS, LSP, XL.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, J., Kang, X., Lu, H. et al. Magnetic Resonance-Guided Focused Ultrasound Thalamotomy Rebalances Atypical Functional Hierarchy in Patients with Essential Tremor. Neurotherapeutics 20, 1755–1766 (2023). https://doi.org/10.1007/s13311-023-01442-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-023-01442-9