Abstract
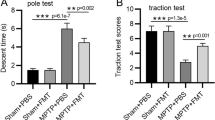
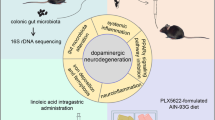
Accumulating data support a crucial role of gut microbiota in Parkinson’s disease (PD). However, gut microbiota vary with age and, thus, will affect PD in an age-dependent, but unknown manner. We examined the effects of fecal microbiota transplantation (FMT) pretreatment, using fecal microbiota from young (7 weeks) or aged mice (23 months), on MPTP-induced PD model. Motor function, pathological changes, striatal neurotransmitters, neuroinflammation, gut inflammation and gut permeability were examined. Gut microbiota composition and metabolites, namely short-chain fatty acids (SCFAs), were analyzed. Neurogenesis was also evaluated by measuring the number of doublecortin-positive (DCX+) neurons and Ki67-positive (Ki67+) cells in the hippocampus. Expression of Cd133 mRNA, a cellular stemness marker, in the hippocampus was also examined. Mice who received FMT from young mice showed MPTP-induced motor dysfunction, and reduction of striatal dopamine (DA), dopaminergic neurons and striatal tyrosine hydroxylase (TH) levels. Interestingly and unexpectedly, mice that received FMT from aged mice showed recovery of motor function and rescue of dopaminergic neurons and striatal 5-hydroxytryptamine (5-HT), as well as decreased DA metabolism after MPTP challenge. Further, they showed improved metabolic profiling and a decreased amount of fecal SCFAs. High-throughput sequencing revealed that FMT remarkably reshaped the gut microbiota of recipient mice. For instance, levels of genus Akkermansia and Candidatus Saccharimonas were elevated in fecal samples of recipient mice receiving aged microbiota (AM + MPTP mice) than YM + MPTP mice. Intriguingly, both young microbiota and aged microbiota had no effect on neuroinflammation, gut inflammation or gut permeability. Notably, AM + MPTP mice showed a marked increase in DCX+ neurons, as well as Ki67+ cells and Cd133 expression in the hippocampal dentate gyrus (DG) compared to YM + MPTP mice. These results suggest that FMT from aged mice augments neurogenesis, improves motor function and restores dopaminergic neurons and neurotransmitters in PD model mice, possibly through increasing neurogenesis.









Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ang QY, Alexander M, Newman JC, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181:1263-1275.e1216. https://doi.org/10.1016/j.cell.2020.04.027.
Desai MS, Seekatz AM, Koropatkin NM, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339-1353.e1321. https://doi.org/10.1016/j.cell.2016.10.043.
Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–52. https://doi.org/10.1038/nri.2016.42.
Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167:915–32. https://doi.org/10.1016/j.cell.2016.10.027.
Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. https://doi.org/10.1126/scitranslmed.3009759.
Kundu P, Lee HU, Garcia-Perez I, et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci Transl Med. 2019;11:eaau4760. https://doi.org/10.1126/scitranslmed.aau4760.
Mosher KI, Wyss-Coray T. Go with your gut: microbiota meet microglia. Nat Neurosci. 2015;18:930–1. https://doi.org/10.1038/nn.4051.
Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469-1480.e1412. https://doi.org/10.1016/j.cell.2016.11.018.
Sun MF, Shen YQ. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res Rev. 2018;45:53–61. https://doi.org/10.1016/j.arr.2018.04.004.
Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–8. https://doi.org/10.1002/mds.26069.
Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179–94. https://doi.org/10.1016/S1474-4422(19)30356-4.
Cersosimo MG, Raina GB, Pecci C, et al. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol. 2013;260:1332–8. https://doi.org/10.1007/s00415-012-6801-2.
Pont-Sunyer C, Hotter A, Gaig C, et al. The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study). Mov Disord. 2015;30:229–37. https://doi.org/10.1002/mds.26077.
Unger MM, Spiegel J, Dillmann KU, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. https://doi.org/10.1016/j.parkreldis.2016.08.019.
Vascellari S, Melis M, Palmas V, et al. Clinical phenotypes of Parkinson’s disease associate with distinct gut microbiota and metabolome enterotypes. Biomolecules. 2021;11:144. https://doi.org/10.3390/biom11020144.
Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30:1351–60. https://doi.org/10.1002/mds.26307.
Torres ERS, Akinyeke T, Stagaman K, et al. Effects of Sub-chronic MPTP exposure on behavioral and cognitive performance and the microbiome of wild-type and mGlu8 knockout female and male mice. Front Behav Neurosci. 2018;12:140. https://doi.org/10.3389/fnbeh.2018.00140.
Fransen F, van Beek AA, Borghuis T, et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. 2017;8:1385. https://doi.org/10.3389/fimmu.2017.01385.
Lee J, d’Aigle J, Atadja L, et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res. 2020;127:453–65. https://doi.org/10.1161/Circresaha.119.316448.
Spychala MS, Venna VR, Jandzinski M, et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. 2018;84:23–36. https://doi.org/10.1002/ana.25250.
D’Amato A, Di Cesare ML, Lucarini E, et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome. 2020;8:140. https://doi.org/10.1186/s40168-020-00914-w.
Cao Q, Qin L, Huang F, et al. Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson’s disease model mice through PI3K/Akt and ERK signaling pathways. Toxicol Appl Pharmacol. 2017;319:80–90. https://doi.org/10.1016/j.taap.2017.01.019.
Perez-Pardo P, Dodiya HB, Engen PA, et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut. 2019;68:829–43. https://doi.org/10.1136/gutjnl-2018-316844.
Zhou ZL, Jia XB, Sun MF, et al. Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson’s disease mice via gut microbiota and mtabolites. Neurotherapeutics. 2019;16:741–60. https://doi.org/10.1007/s13311-019-00719-2.
Garcia-Villalba R, Gimenez-Bastida JA, Garcia-Conesa MT, Tomas-Barberan FA, Carlos Espin J, Larrosa M. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples. J Sep Sci. 2012;35:1906–13. https://doi.org/10.1002/jssc.201101121.
Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–6. https://doi.org/10.1126/science.aao5774.
Nagpal R, Mainali R, Ahmadi S, et al. Gut microbiome and aging: Physiological and mechanistic insights. Nutr Healthy Aging. 2018;4:267–85. https://doi.org/10.3233/NHA-170030.
Liu YQ, Hu XY, Zheng W, et al. Action mechanism of hypoglycemic principle 9-(R)-HODE isolated from cortex lycii based on a metabolomics approach. Front Pharmacol. 2022. https://doi.org/10.3389/fphar.2022.1011608.
Tian BM, Zhao JH, Zhang M, et al. Lycium ruthenicum anthocyanins attenuate high-fat diet-induced colonic barrier dysfunction and inflammation in mice by modulating the gut microbiota. Mol Nutr Food Res. 2021. https://doi.org/10.1002/mnfr.202000745.
Lopez-Montoya P, Cerqueda-Garcia D, Rodriguez-Flores M, et al. Association of gut microbiota with atherogenic dyslipidemia, and its impact on serum lipid levels after bariatric surgery. Nutrients. 2022. https://doi.org/10.3390/nu14173545.
Zhuge A, Li S, Yuan Y, Li B, Li L. The synergy of dietary supplements Lactobacillus salivarius LI01 and Bifidobacterium longum TC01 in alleviating liver failure in rats treated with D-galactosamine. Food Funct. 2021;12:10239–52. https://doi.org/10.1039/d1fo01807h.
Amabebe E, Robert FO, Agbalalah T, Orubu ESF. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br J Nutr. 2020;123:1127–37. https://doi.org/10.1017/S0007114520000380.
O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. https://doi.org/10.1016/j.bbr.2014.07.027.
Jang JH, Yeom MJ, Ahn S, et al. Acupuncture inhibits neuroinflammation and gut microbial dysbiosis in a mouse model of Parkinson’s disease. Brain Behav Immun. 2020;89:641–55. https://doi.org/10.1016/j.bbi.2020.08.015.
Sun MF, Zhu YL, Zhou ZL, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav Immun. 2018;70:48–60. https://doi.org/10.1016/j.bbi.2018.02.005.
Singh R, Chandrashekharappa S, Bodduluri SR, et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019;10:89. https://doi.org/10.1038/s41467-018-07859-7.
Kim N, Jeon SH, Ju IG, et al. Transplantation of gut microbiota derived from Alzheimer’s disease mouse model impairs memory function and neurogenesis in C57BL/6 mice. Brain Behav Immun. 2021;98:357–65. https://doi.org/10.1016/j.bbi.2021.09.002.
Wei GZ, Martin KA, Xing PY, et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2021;118:e2021091118. https://doi.org/10.1073/pnas.2021091118.
Ma XY, Xiao WC, Li H, et al. Metformin restores hippocampal neurogenesis and learning and memory via regulating gut microbiota in the obese mouse model. Brain Behav Immun. 2021;95:68–83. https://doi.org/10.1016/j.bbi.2021.02.011.
Ryu S, Jeon H, Koo S, Kim S. Korean red ginseng enhances neurogenesis in the subventricular zone of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice. Front Aging Neuroscience. 2018;10:355. https://doi.org/10.3389/fnagi.2018.00355.
Zhao L, Zhang Q, Ma W, Tian F, Shen H, Zhou M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017;8:4644–56. https://doi.org/10.1039/c7fo01383c.
Hu L, Jin L, Xia D, et al. Nitrate ameliorates dextran sodium sulfate-induced colitis by regulating the homeostasis of the intestinal microbiota. Free Radical Biol Med. 2020;152:609–21. https://doi.org/10.1016/j.freeradbiomed.2019.12.002.
Tan FHP, Liu G, Lau SA, et al. Lactobacillus probiotics improved the gut microbiota profile of a Drosophila melanogaster Alzheimer’s disease model and alleviated neurodegeneration in the eye. Benef Microbes. 2020;11:79–89. https://doi.org/10.3920/BM2019.0086.
Yang X, Yu D, Xue L, Li H, Du J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm Sin B. 2020;10:475–87. https://doi.org/10.1016/j.apsb.2019.07.001.
Li Y, Ning L, Yin Y, et al. Age-related shifts in gut microbiota contribute to cognitive decline in aged rats. Aging (Albany NY). 2020;12:7801–17. https://doi.org/10.18632/aging.103093.
Cuervo-Zanatta D, Garcia-Mena J, Perez-Cruz C. Gut microbiota alterations and cognitive impairment are sexually dissociated in a transgenic mice model of Alzheimer’s disease. J Alzheimers Dis. 2021;82:S195–214. https://doi.org/10.3233/JAD-201367.
Bhattarai Y, Si J, Pu M, et al. Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson’s disease. Gut Microbes. 2021;13:1866974. https://doi.org/10.1080/19490976.2020.1866974.
Zhou XT, Lu JC, Wei KH, et al. Neuroprotective effect of ceftriaxone on MPTP-induced Parkinson’s disease mouse model by regulating inflammation and intestinal microbiota. Oxid Med Cell Longev. 2021;2021:9424582. https://doi.org/10.1155/2021/9424582.
Bian XY, Wu WR, Yang LY, et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol. 2019;10:2259. https://doi.org/10.3389/fmicb.2019.02259.
Yang YJ, Zhong ZQ, Wang BJ, et al. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology. 2019;44:2054–64. https://doi.org/10.1038/s41386-019-0437-1.
Blacher E, Bashiardes S, Shapiro H, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572:474–80. https://doi.org/10.1038/s41586-019-1443-5.
Luo LM, Luo JL, Cai YT, et al. Inulin-type fructans change the gut microbiota and prevent the development of diabetic nephropathy. Pharmacol Res. 2022;183:106367. https://doi.org/10.1016/j.phrs.2022.106367.
Yan ZZ, Yang F, Cao JW, et al. Alterations of gut microbiota and metabolome with Parkinson’s disease. Microb Pathog. 2021;160:105187. https://doi.org/10.1016/j.micpath.2021.105187.
Qiao CM, Sun MF, Jia XB, et al. Sodium butyrate exacerbates Parkinson’s disease by aggravating neuroinflammation and colonic inflammation in MPTP-induced mice model. Neurochem Res. 2020;45:2128–42. https://doi.org/10.1007/s11064-020-03074-3.
Bagetta V, Ghiglieri V, Sgobio C, Calabresi P, Picconi B. Synaptic dysfunction in Parkinson’s disease. Biochem Soc Trans. 2010;38:493–7. https://doi.org/10.1042/BST0380493.
Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B. Levodopa-induced dyskinesias in patients with Parkinson’s disease: filling the bench-to-bedside gap. Lancet Neurol. 2010;9:1106–17. https://doi.org/10.1016/S1474-4422(10)70218-0.
Jyothi HJ, Vidyadhara DJ, Mahadevan A, et al. Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol Aging. 2015;36:3321–33. https://doi.org/10.1016/j.neurobiolaging.2015.08.024.
Erny D, Hrabe de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–77. https://doi.org/10.1038/nn.4030.
Apple DM, Solano-Fonseca R, Kokovay E. Neurogenesis in the aging brain. Biochem Pharmacol. 2017;141:77–85. https://doi.org/10.1016/j.bcp.2017.06.116.
Marchetti B, Tirolo C, L’Episcopo F, et al. Parkinson’s disease, aging and adult neurogenesis: Wnt/β-catenin signalling as the key to unlock the mystery of endogenous brain repair. Aging Cell. 2020;19:e13101. https://doi.org/10.1111/acel.13101.
Winner B, Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2015;7:021287. https://doi.org/10.1101/cshperspect.a021287.
Hoglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–35. https://doi.org/10.1038/nn1265.
Singh S, Mishra A, Mishra SK, Shukla S. ALCAR promote adult hippocampal neurogenesis by regulating cell-survival and cell death-related signals in rat model of Parkinson’s disease like-phenotypes. Neurochem Int. 2017;108:388–96. https://doi.org/10.1016/j.neuint.2017.05.017.
Zhang T, Hong J, Di T, Chen L. MPTP impairs dopamine D1 receptor-mediated survival of newborn neurons in ventral hippocampus to cause depressive-like behaviors in adult mice. Front Mol Neurosci. 2016;9:101. https://doi.org/10.3389/fnmol.2016.00101.
Acknowledgements
This study was supported by National Natural Science Foundation of China (81771384, 81801276, 82171429), Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX19 1893), Public Health Research Center at Jiangnan University (JUPH201801), Youth Foundation of Basic Research Program of Jiangnan University in 2021 (JUSRP121063). We are grateful to Dr. Stanley Li Lin for his critical revision on the manuscript, both on language and science.
Author information
Authors and Affiliations
Contributions
The experiment design and management: Chen-Meng Qiao and Yan-Qin Shen; Animal experiment operation: Chen-Meng Qiao, Yu-Zhou, Wei Quan, Gu-Yu Niu and Xiao-Yu Ma; Tissue collection: Chen-Meng Qiao, Yu-Zhou, Wei Quan, Gu-Yu Niu, Yun Shi, Li-Ping Zhao, Xiao-Yu Ma, Hui Hong and Jian Wu; HPLC/16S rRNA: Chen-Meng Qiao, Yu Zhou and Wei Quan; Quantitative real-time PCR analysis: Yu Zhou and Wei Quan; IF/IHC and image analysis: Yu Zhou, Wei Quan, Chen-Meng Qiao, Xiao-Yu Ma and Wei-Jiang Zhao; Western bolt: Yu Zhou and Wei Quan; Nissl staining: Chen-Meng Qiao, Wei Quan and Xiao-Yu Ma; Statistical analysis: Chen-Meng Qiao and Yu Zhou; Writing and revising of manuscript: Chen-Meng Qiao and Yan-Qin Shen.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiao, CM., Zhou, Y., Quan, W. et al. Fecal Microbiota Transplantation from Aged Mice Render Recipient Mice Resistant to MPTP-Induced Nigrostriatal Degeneration Via a Neurogenesis-Dependent but Inflammation-Independent Manner. Neurotherapeutics 20, 1405–1426 (2023). https://doi.org/10.1007/s13311-023-01420-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-023-01420-1