Abstract
Delayed cerebral ischemia (DCI) is a serious complication of aneurysmal subarachnoid hemorrhage (SAH). Matricellular protein periostin (POSTN) has been found to be upregulated and linked with early brain injury after experimental SAH. The aim of the present study was to investigate the relationship between plasma POSTN levels and various clinical factors including serum levels of C-reactive protein (CRP), an inflammatory marker, in 109 consecutive SAH patients whose POSTN levels were measured at days 1–12 after aneurysmal obliteration. DCI developed in 16 patients associated with higher incidence of angiographic vasospasm, cerebral infarction, and 90-day worse outcomes. POSTN levels peaked at days 4–6 before DCI development. Cerebrospinal fluid (CSF) drainage was associated with reduced POSTN levels, but did not influence CRP levels. There was no correlation between POSTN levels and other treatments or CRP levels. To predict DCI development, receiver-operating characteristic curves indicated that the most reasonable cutoff POSTN levels were obtained at days 1–3 in patients without CSF drainage (80.5 ng/ml; specificity, 77.6%; sensitivity, 85.7%). Multivariate analyses using variables obtained by day 3 revealed that POSTN level was an independent predictor of DCI. POSTN levels over the cutoff value were associated with higher incidence of DCI, but not angiographic vasospasm. This study shows for the first time that CSF drainage may reduce plasma POSTN levels, and that POSTN levels may increase prior to the development of DCI with and without vasospasm irrespective of systemic inflammatory reactions in clinical settings. These findings suggest POSTN as a new therapeutic molecular target against post-SAH DCI.
Similar content being viewed by others
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is a life-threatening disease even in these years [1]. Patients who survive an early stage of aneurysmal SAH and have their aneurysms obliterated are still at risk of delayed cerebral ischemia (DCI) that often results in severe disabilities or death [1,2,3]. The mechanisms of post-SAH brain injury are multifactorial, but neuroinflammation is considered as an important cause [4,5,6,7,8,9].
Periostin (POSTN) is a 90-kDa extracellular matrix protein belonging to the fasciclin family, which was originally reported as osteoblast-specific factor 2 [10]. POSTN is closely related to inflammation and modulates immune and nonimmune cells as a matricellular protein [11]. Therefore, POSTN has been paid attention to as a biomarker or a therapeutic target of inflammatory diseases [11, 12]. Recently, we reported that POSTN was upregulated in cerebral cortex after SAH in mice and was responsible for early brain injury (EBI) in terms of blood–brain barrier (BBB) disruption [13]. There has been only one paper published in a clinical setting, in which admission and pretreatment peripheral blood levels of POSTN were related to clinical severity, DCI, and poor outcomes after aneurysmal SAH [14]. However, it remains unknown how peripheral blood POSTN levels change with time, and if various treatments that are performed for aneurysmal obliteration or prevention of DCI influence peripheral blood POSTN levels. More importantly, it is still unclear if peripheral blood levels of POSTN increase in association with the development of cerebral vasospasm or other neurovascular causes of DCI, or are merely a ubiquitous or confounding factor reflecting systemic inflammation. In the present study, thus, we measured plasma POSTN levels chronologically from the day postaneurysmal obliteration to 12 days post-SAH and investigated the relationship between plasma POSTN levels and various clinical factors including vasospasm, DCI, treatment, and serum levels of C-reactive protein (CRP) as a nonspecific systemic inflammatory marker.
Methods
The Institutional Ethics Committee approved the study, and written informed consent was obtained from the relatives.
Study Population
The present study included consecutive patients registered in the prospective registry for searching mediators of neurovascular events after aneurysmal SAH (pSEED) [15, 16] that was conducted in 9 tertiary referral centers in Mie prefecture in Japan (listed in Supplementary Material: Appendix) from September 2013 to May 2015. In the registry, in addition to clinical variables, plasma samples were serially collected and stored at − 78 °C. In the present study, plasma POSTN levels were measured using the stocked plasma samples, and the relationship between plasma POSTN levels and clinical variables was retrospectively analyzed. The inclusion criteria were as follows: ≥ 20 years of age at onset, modified Rankin scale (mRS) 0–2 before onset, SAH on computed tomography (CT) or lumbar puncture, saccular aneurysm as the cause of SAH confirmed on CT angiography or digital subtraction angiography, and aneurysmal obliteration by clipping or coil embolization within 48 h of onset. The exclusion criteria were as follows: unknown etiology or other causes of SAH such as ruptured fusiform, dissecting, traumatic, mycotic and arteriovenous malformation-related aneurysms, treatment by parent artery occlusion without bypass surgery, angiographic or treatment-related complications such as cerebral infarction or hemorrhage, serious premorbidities such as heart, respiratory, or renal failure that precluded enough anti-DCI treatment, and concomitant inflammatory diseases that are known to upregulate POSTN [11]. Timing, treatment modality (clipping or coil embolization) of aneurysmal obliteration, and other medical management or treatment strategies depended on the onsite investigators.
Clinical Variables
Baseline demographic and clinical data included age, sex, existing co-morbidities (hypertension, dyslipidemia, and diabetes mellitus; previously diagnosed or on medication), current smoking, preoperative World Federation of Neurological Surgeons (WFNS) grade, modified Fisher grade [17] obtained by CT on admission, acute hydrocephalus, and the location of a ruptured aneurysm (anterior or posterior circulation). Acute hydrocephalus was recorded when there was an evidence of ventriculomegaly proved by CT on admission, which was considered to cause neurological impairments such as disturbance of consciousness: a catheter of cerebrospinal fluid (CSF) drainage was placed at clipping or the day after coiling if ventriculomegaly was continued. As treatment-related variables, the following factors were evaluated: treatment modality (clipping or coil embolization) performed for aneurysmal obliteration, CSF drainage including ventricular, cisternal and spinal drainage to manage hydrocephalus and/or to promote hematoma clearance, medication to prevent DCI (intravenously administered fasudil hydrochloride, oral or enteral cilostazol, eicosapentaenoic acid [EPA], and statin), and rescue therapies for DCI (intentional hypertension, and intra-arterial injections of fasudil hydrochloride). Outcomes were assessed as to DCI, angiographic vasospasm, cerebral infarction, and mRS at 90 days post-SAH. DCI was defined as otherwise unexplained clinical deterioration (i.e., focal neurological deficits, a decrease of at least 2 points on the Glasgow Coma Scale, or both), which lasted for at least 1 h [1]. Other potential causes of clinical deterioration were excluded on clinical assessment, CT, magnetic resonance images, or laboratory studies. Angiographic vasospasm was defined as 50% or more reduction in the baseline vessel diameter of major cerebral arteries on CT angiography or digital subtraction angiography irrespective of symptoms, which was performed around 7 days post-SAH or at neurological deterioration. Cerebral infarction was defined as newly developed infarct after aneurysmal obliteration on CT images that was judged not to be iatrogenic, and occurred within 1 month after SAH. Determination of these factors was made at each center, and the organizing committee qualified them.
Measurement of Plasma POSTN
After aneurysmal obliteration, blood samples were serially collected with minimal stasis from a vein at days 1–3, 4–6, 7–9, and 10–12 after onset. All samples were centrifuged for 5 min at 3000 G, and the supernatants were stored at − 78 °C until assayed. Plasma POSTN levels were determined using a commercially available enzyme-linked immunosorbent assay kit for human POSTN (AG-45B-0004-KI01; Adipogen, Switzerland).
As a control, blood samples were also obtained from 6 patients with unruptured cerebral aneurysms who provided written informed consent (mean, 65.2 years old; female/male = 1/5) before any invasive procedure, and plasma POSTN levels were determined as described above.
Serum CRP
To determine the relationship between plasma POSTN levels and serum levels of CRP, CRP data were retrospectively collected and analyzed. CRP levels were measured with an automatic chemistry analyzer using the same blood samples as that for POSTN measurements at each center.
Volume of CSF Drainage
In patients with CSF drainage, the duration and the volume of CSF output were retrospectively collected. For every POSTN level, the total volume of CSF drainage was obtained as the total CSF drainage volume by the POSTN measurement point, respectively. Daily drained volume of CSF was calculated by dividing the total volume of CSF drainage (ml) by the duration from the day of aneurysmal obliteration to that of blood sampling for the POSTN measurement (day): if there was the duration of no CSF drainage, the volume of CSF drainage during the period was calculated as 0 ml.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or standard error of the mean (SEM; for graphs) and were compared between groups by unpaired t tests after confirming that each population being compared followed a normal distribution using Shapiro–Wilk W tests. Categorical variables were reported as percentage and were compared using chi-square or Fisher’s exact tests. Correlation between plasma POSTN levels and various factors was evaluated by Pearson’s correlation coefficient for continuous variables or point biserial correlation coefficient for categorical variables. Receiver-operating characteristic (ROC) curves and the area under the curve (AUC) were analyzed for plasma POSTN levels and subsequent DCI development. Multivariate logistic regression analyses with DCI as the dependent variable were performed by the backward stepwise method using all variables except for outcome variables. For multivariate analyses, WFNS grade was divided into grades I–III and IV–V, and modified Fisher grade was categorized as grades 1–3 and 4. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics [18]. A significant level was set at p value < 0.05.
Results
Comparison Between Patients With and Without DCI
One hundred and thirty-six consecutive patients were registered in this period. In total, 109 patients were included in the present study and 27 patients were excluded (10 patients, no blood samples; 9 patients, dissecting aneurysms treated by parent artery occlusion; 4 patients, aneurysmal obliteration after 48 h of onset; and 4 patients, preonset mRS ≥ 3) (Supplementary Material: Fig. S1). Plasma POSTN levels at days 1–3 and 4–6 were measured in all patients, but those at days 7–9 and 10–12 were missing in one patient, respectively. In addition, the following data were missing in some cases: current smoking (13 patients), acute hydrocephalus (1 patient), and angiographic vasospasm (5 patients). The analyses were performed except for the missing data. The mean age of patients was 65.2 ± 12.6 (SD) years old, and 77 cases (70.6%) were female. The number of patients with preoperative WFNS grades IV–V was 39 (35.8%). DCI developed in 16 cases (days 1–3, 0 case; days 4–6, 3 cases; days 7–9, 9 cases; days 10–12, 2 cases; and day 13 and later, 2 cases). Age, sex, co-morbidities (hypertension, dyslipidemia, and diabetes mellitus), current smoking, preoperative WFNS grades, modified Fisher grades, acute hydrocephalus, and ruptured aneurysm location were not different between patients with and without DCI (Table 1). Treatment modality (clipping or coiling), CSF drainage, and anti-DCI preventive medications (intravenous fasudil hydrochloride, cilostazol, EPA, and stain) were also similar between the 2 groups. However, patients with DCI were associated with significantly higher incidence of angiographic vasospasm, cerebral infarction, and 90-day worse outcomes, although intentional hypertension was performed in 5 patients developing DCI (31.3% vs 3.2% in no-DCI group, p = 0.002) and intra-arterial injections of fasudil hydrochloride were performed in 7 patients developing DCI (43.8% vs 1.1% in no-DCI group, p < 0.001).
Relationship Between Plasma POSTN Levels and DCI, Other Clinical Variables or CRP
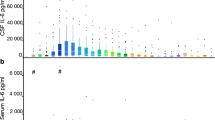
Compared with plasma POSTN levels in control cases (52.3 ± 4.0 [SEM] ng/ml), plasma levels of POSTN in aneurysmal SAH patients were higher. Plasma POSTN levels peaked at days 4–6, when the levels were significantly higher in patients with subsequent DCI development compared with those without DCI (Fig. 1a). Serum CRP levels were measured in 86 patients at days 1–3, 84 patients at days 4–6, 83 patients at days 7–9, and 84 patients at days 10–12: CRP levels were not significantly different between patients with and without DCI at any sampling point (Fig. 1b). When correlation was examined between post-SAH plasma POSTN levels and clinical variables, plasma POSTN levels at days 4–6 were weakly but positively correlated with DCI and the resultant intentional hypertension. In contrast, weak and negative correlation was observed between plasma POSTN levels at days 1–3 and preoperative WFNS grades or acute hydrocephalus, and between plasma POSTN levels at days 1–3 to days 10–12 and CSF drainage (Supplementary Material: Table S1). Especially, plasma POSTN levels were significantly lower in patients with than without CSF drainage at any sampling point (Fig. 2a). The treatment modality of aneurysmal obliteration, angiographic vasospasm, cerebral infarction, and 90-day outcomes were not correlated with plasma POSTN levels (Supplementary Material: Table S1).
Chronological comparison of plasma periostin levels (a) and serum C-reactive protein (CRP) levels (b) between patients with and without delayed cerebral ischemia (DCI). Periostin levels are significantly higher in patients with DCI compared with those without DCI at days 4–6 (*p < 0.05 vs no DCI; unpaired t test). CRP levels are not significantly different between the DCI and the no-DCI groups. Data, mean ± standard error of the mean; ( ), number of patients
Chronological comparison of plasma periostin levels (a) and serum C-reactive protein (CRP) levels (b) between patients with and without cerebrospinal fluid (CSF) drainage. Periostin levels are significantly lower in patients with CSF drainage at any sampling point, while CRP levels are significantly higher in patients with CSF drainage at days 7–9 (*p < 0.05, **p < 0.001 vs no CSF drainage; unpaired t test). Data, mean ± standard error of the mean; ( ), number of patients
CSF drainage was performed in 53 patients (48.6%; Supplementary Material: Fig. S1) and the duration was 4.9 ± 4.1 (SD) days (range, 1–12). The volume of CSF drainage was missing in 2 cases; in addition, 15 cases underwent continuous cisternal irrigation by artificial CSF, and therefore, the precise CSF output volume could not be measured in those patients. Thus, data in the remaining patients were used to evaluate the relationship between plasma POSTN levels and the CSF drainage volume at each period: both daily drained volume of CSF and the total volume of CSF drainage were negatively correlated with plasma POSTN levels (Supplementary Material: Fig. S2).
CRP levels were not correlated with plasma POSTN levels at any sampling point (Supplementary Material: Table S1). In contrast to POSTN levels, CRP levels tended to be higher in patients with CSF drainage: CRP levels were significantly higher in patients with than without CSF drainage at days 7–9 (Fig. 2b).
ROC Curve Analyses for the Performance of Plasma POSTN to Predict DCI
The ROC curve analyses for the performance of plasma POSTN levels were separately performed in patients with CSF drainage and in those without CSF drainage (Supplementary Material: Fig. S1). To predict the development of DCI in patients without CSF drainage, the ROC curve indicated that plasma POSTN with a cutoff value of 80.5 ng/ml resulted in a specificity of 77.6% and a sensitivity of 85.7% at days 1–3 (AUC, 0.770; 95% confidence interval [CI], 0.581–0.958), and that the specificity was 65.3% and sensitivity was 85.7% when a cutoff value of 71.3 ng/ml was set at days 4–6 (AUC, 0.770; 95% CI, 0.622–0.917; Fig. 3). However, at both days 7–9 and 10–12, no reasonable cutoff value was obtained. In patients with CSF drainage, no reasonable cutoff value of plasma POSTN was obtained to predict DCI at any sampling point (Supplementary Material: Fig. S3).
Receiver-operating characteristic curve for the performance of plasma periostin at each sampling point according to the presence versus the absence of delayed cerebral ischemia after subarachnoid hemorrhage in patients with no cerebrospinal fluid drainage. AUC = area under the curve, CI = confidence interval
Univariate Analyses for DCI Development in Patients With or Without CSF Drainage
When CSF drainage was not performed, DCI development was significantly associated with more amount of SAH assessed by modified Fisher grade (especially higher incidence of grade 4; Table 2) and higher plasma POSTN levels at days 1–3 and 4–6 (Fig. 4a). CRP levels were also significantly higher in patients with DCI at days 4–6 (Fig. 4b). Although treatments other than intentional hypertension were not significantly different between patients with and without DCI, patients with DCI had significantly higher incidence of intentional hypertension, angiographic vasospasm, cerebral infarction, and worse 90-day outcomes (Table 2).
Chronological comparison of plasma periostin levels (a) and serum C-reactive protein (CRP) levels (b) between patients with and without delayed cerebral ischemia (DCI) when cerebrospinal fluid drainage was not performed. Periostin levels at days 1–3 and 4–6, and CRP levels at days 4–6 are significantly higher in patients with DCI (*p < 0.05 vs no DCI; unpaired t test). Data, mean ± standard error of the mean; ( ), number of patients
CSF drainage was more frequently performed in patients with worse clinical grades (WFNS grades IV-V, 54.7% vs 17.9% in no CSF drainage, p < 0.001 and modified Fisher grade 4, 50.9% vs 25.0% in no CSF drainage, p = 0.009) and acute hydrocephalus (62.3% vs 18.2% in no CSF drainage, p < 0.001), but the incidence of DCI in patients with CSF drainage was similar to those without CSF drainage (Supplementary Material: Table S2). In patients with CSF drainage, the difference in the amount of SAH assessed by modified Fisher grade (Table 2), plasma POSTN levels, and serum CRP levels (Supplementary Material: Fig. S4) was not detected between patients with and without DCI. Patients with DCI had higher incidence of angiographic vasospasm and cerebral infarction, but 90-day outcomes were not significantly different between patients with and without DCI (Table 2).
Multivariate Analyses for DCI Development
To reduce potential selection bias as much as possible, variables for multivariate logistic regression analyses were selected by the downward stepwise method using all variables that were obtained by day 3 post-SAH, because most of variables including WFNS grade were not significant on univariate analyses (Tables 1 and 2) and correlation between variables was weak (Supplementary Material: Table S1). The analyses revealed that plasma POSTN level at days 1–3 as a continuous variable was the sole independent predictor of the subsequent development of DCI when assessed in all patients (odds ratio [OR], 1.030; 95% CI, 1.000–1.060; p = 0.034; Supplementary Material: Table S3). When multivariate logistic regression analyses were performed in patients without CSF drainage, plasma POSTN levels at days 1–3 were also an independent predictor of the subsequent development of DCI: the OR was 23.800 (95% CI, 1.900–299.000; p = 0.014) when plasma POSTN levels at days 1–3 were categorized using the cutoff value of 80.5 ng/ml determined in Fig. 3 (Table 3), and the OR was 1.050 (95% CI, 1.000–1.100; p = 0.031) when plasma POSTN levels were used as a continuous variable (Supplementary Material: Table S4). When multivariate logistic regression analyses were performed in patients with CSF drainage, plasma POSTN levels at days 1–3 were not an independent predictor of DCI (Supplementary Material: Table S5).
Clinical Significance of Elevated Plasma POSTN Levels
In patients with no CSF drainage, the relationship between plasma POSTN levels over the cutoff value determined in Fig. 3 and clinical variables or outcome measures was examined (Supplementary Material: Table S6). Higher POSTN levels were associated with higher incidence of DCI, but not related with angiographic vasospasm, cerebral infarction, 90-day outcomes as well as serum CRP levels at both days 1–3 and 4–6.
Discussion
The novel findings of the present study were that: 1) plasma POSTN levels kept high at days 1–12 and peaked at days 4–6; 2) plasma POSTN levels were significantly lower in patients with CSF drainage compared with those without CSF drainage, although CSF drainage was performed in patients with poorer initial clinical status; 3) more CSF drainage volume was associated with lower plasma POSTN levels; 4) plasma POSTN levels were significantly higher in patients with than without DCI at days 1–3 and 4–6 when CSF drainage was not performed, but CSF drainage lost the difference; 5) treatments other than CSF drainage did not affect plasma POSTN levels before onset of DCI; 6) plasma POSTN levels were not correlated with serum CRP levels; 7) plasma POSTN levels at days 1–3 was an independent predictor of DCI in patients without CSF drainage; and 8) plasma POSTN levels were not related to angiographic vasospasm. These findings suggest that plasma POSTN increases prior to the development of DCI with and without angiographic vasospasm. In addition, considering the findings that CSF drainage precluded the elevation of plasma POSTN levels irrespective of a nonspecific systemic inflammatory marker CRP level, POSTN might be produced in the intracranial space. Plasma POSTN levels may reflect an intracranial pathophysiology, and those at days 1–3 may be useful to predict DCI regardless of vasospasm in patients without CSF drainage.
Under normal conditions, POSTN is present at low levels in adult tissues, but highly expressed at sites of inflammation or injury [19]. POSTN is characterized as a molecule easily moving or secreted from inflamed sites into various body fluids such as blood [20], urine [21], sputum [22], and tears [23], although the mechanism of the moving or secretion still remains unclear [11]. Although it is not certain whether POSTN is upregulated in post-SAH injured brain, and then secreted into peripheral blood in a clinical setting, BBB disruption may influence the permeability of POSTN. POSTN is a 90-kDa protein, which is much bigger than ≤ 500 Da molecules that pass BBB easily [24]. In patients with SAH, however, BBB disruption occurs [25], possibly allowing POSTN to move from brain or CSF to peripheral blood, or vice versa. Actually, the present study showed that more CSF drainage volume was significantly correlated with reduced plasma POSTN levels without influencing serum CRP levels. These findings suggest that POSTN might be induced in post-SAH brain or CSF corresponding with our recent experimental study, in which POSTN was upregulated in brain capillary endothelial cells and neurons in a filament perforation SAH model in mice [13]. CSF drainage might decrease the amount of POSTN that could move from the brain and CSF to the peripheral blood in this study. In contrast, as CRP is synthesized in liver and increases by nonspecific inflammatory reactions [26], CSF drainage could not reduce serum CRP levels.
The present study revealed that plasma POSTN levels significantly increased before the development of DCI but not associated with angiographic vasospasm in patients without CSF drainage. ROC curve analyses suggested that plasma POSTN levels at days 1–3 and 4–6 could be used as a biomarker for predicting DCI in patients without CSF drainage. The underlying mechanism of DCI development has not been clarified exactly, and nonvasospastic causes of DCI have been attracted attention to recently [4, 27, 28]. However, inflammation is considered to be an important cause of DCI irrespective of vasospasm [4, 27,28,29,30]. As tissue injury and inflammation induce the expression of POSTN, post-SAH neuroinflammation may cause POSTN upregulation [11, 19]. In experimental SAH, POSTN caused EBI or BBB disruption via the activation of mitogen-activated protein kinases (MAPKs) and matrix metalloproteinase (MMP)-9, interacting with another matricellular protein tenascin-C [13]. MAPKs and MMP-9 are well known to cause the degradation of cerebral microvessel basal lamina and tight junction protein zona occludens-1, resulting in BBB disruption and brain edema formation after SAH [31, 32]. A more recent study also showed the possible involvement of POSTN in the development of EBI or BBB disruption after experimental SAH [28]. Thus, post-SAH increases in POSTN may cause EBI, which in turn may lead to DCI with no vasospasm [13]. In fact, a previous clinical study reported positive correlation between admission WFNS grade, which may reflect the severity of EBI, and pretreatment peripheral blood POSTN levels [14]. In the present study, admission WFNS grade and plasma POSTN levels at days 1–3 were negatively correlated, but this may be because poor-grade patients more frequently underwent CSF drainage, resulting in lower plasma POSTN levels. On the other hand, CSF drainage reduced plasma POSTN levels that were not different between patients with and without DCI, but DCI still developed in the present study. This can be explained by the speculation that CSF drainage reduced plasma POSTN levels through preventing POSTN from moving to peripheral blood from the central nervous system, but POSTN levels in the CSF and brain tissues might remain high, causing DCI.
In human and mouse, POSTN is known to undergo alternative splicing between exons 16 and 22 in its C-terminal region, and expressed as specific splicing isoforms, which may have different functions depending on a disease [33]. To date, 9 splicing variants have been identified, but the function of different splicing variants has not been fully understood [34]. At present, clinically important or well-known variants are as follows: POSTN 1, a full-length form containing 23 exons; POSTN 2, which lacks exon 17; POSTN 3, which lacks exon 21; and POSTN 4, which lacks exons 17 and 21 [34]. For example, POSTN 2 exhibited neuroprotective effects and accelerated neurite outgrowth in transient focal cerebral ischemia in mice associated with Akt phosphorylation [35]. On the other hand, in experimental SAH in mice, POSTN 1 caused BBB disruption and brain edema formation [13]. However, it remains unknown whether splicing variants of POSTN other than POSTN 1 are upregulated after SAH, and if so, how different splicing variants of POSTN function after SAH. According to the manufacturer’s datasheet (https://www.biomol.com/product_Periostin-human-ELISA-Kit.html?aRelated=AG-45B-0004), the assay kit used in the present study can detect POSTN isoforms 1–4 together, but not separately. As splicing variants of POSTN may have opposite functions, thus, further studies are needed to investigate the time course of each variant of POSTN induction and the relationship among each POSTN splicing variant and post-SAH neurovascular events including DCI.
There are several limitations in the present study. First, the present study used a prospectively maintained pSEED database, but this is a small-scale retrospective study with potential selection bias. To confirm the findings in the present study, therefore, large-scale prospective studies are needed. Second, CSF levels of POSTN were not measured. In patients with CSF drainage, it may be useful to measure POSTN levels in both peripheral blood and CSF simultaneously to confirm whether POSTN is induced in the central nervous system or merely reflects system inflammatory reactions. Third, plasma POSTN levels were not compared between before and after CSF drainage to more clearly show effects of CSF drainage on plasma POSTN levels. Fourth, the method used in the present study could not distinguish POSTN isoforms as described above. However, the present study showed for the first time that post-SAH induction of POSTN might precede the development of DCI regardless of vasospasm in a clinical setting, and suggests the possibility of POSTN as a new therapeutic target against DCI not directly related to vasospasm. Further studies would prove the significance and usefulness of POSTN researches in DCI after SAH.
References
Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010;41:2391–2395. https://doi.org/10.1161/strokeaha.110.589275
Suzuki H, Nakatsuka Y, Yasuda R, et al. Dose-dependent inhibitory effects of cilostazol on delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. Transl Stroke Res 2018. https://doi.org/10.1007/s12975-018-0650-y
Suzuki H, Shiba M, Nakatsuka Y, Nakano F, Nishikawa H. Higher cerebrospinal fluid pH may contribute to the development of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Transl Stroke Res 2017;8:165–173. https://doi.org/10.1007/s12975-016-0500-8
Okada T, Suzuki H. Toll-like receptor 4 as a possible therapeutic target for delayed brain injuries after aneurysmal subarachnoid hemorrhage. Neural Regen Res 2017;12:193–196. https://doi.org/10.4103/1673-5374.200795
Atangana E, Schneider UC, Blecharz K, et al. Intravascular inflammation triggers intracerebral activated microglia and contributes to secondary brain injury after experimental subarachnoid hemorrhage (eSAH). Transl Stroke Res 2017;8:144–156. https://doi.org/10.1007/s12975-016-0485-3
Nishikawa H, Suzuki H. Possible role of inflammation and galectin-3 in brain injury after subarachnoid hemorrhage. Brain Sci 2018;8:E30. https://doi.org/10.3390/brainsci8020030
de Oliveira Manoel AL, Macdonald RL. Neuroinflammation as a target for intervention in subarachnoid hemorrhage. Front Neurol 2018;9:292. https://doi.org/10.3389/fneur.2018.00292
Zheng VZ, Wong GKC. Neuroinflammation responses after subarachnoid hemorrhage: a review. J Clin Neurosci 2017;42:7–11. https://doi.org/10.1016/j.jocn.2017.02.001
Lucke-Wold BP, Logsdon AF, Manoranjan B, et al. Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int J Mol Sci 2016;17:497. https://doi.org/10.3390/ijms17040497
Hu F, Shang XF, Wang W, et al. High-level expression of periostin is significantly correlated with tumour angiogenesis and poor prognosis in osteosarcoma. Int J Exp Pathol 2016;97:86–92. https://doi.org/10.1111/iep.12171
Izuhara K, Nunomura S, Nanri Y, et al. Periostin in inflammation and allergy. Cell Mol Life Sci 2017;74:4293–4303. https://doi.org/10.1007/s00018-017-2648-0
Suzuki H, Nishikawa H, Kawakita F. Matricellular proteins as possible biomarkers for early brain injury after aneurysmal subarachnoid hemorrhage. Neural Regen Res 2018;13:1175–1178. https://doi.org/10.4103/1673-5374.235022
Liu L, Kawakita F, Fujimoto M, et al. Role of periostin in early brain injury after subarachnoid hemorrhage in mice. Stroke 2017;48:1108–1111. https://doi.org/10.1161/strokeaha.117.016629
Luo W, Wang H, Hu J. Increased concentration of serum periostin is associated with poor outcome of patients with aneurysmal subarachnoid hemorrhage. J Clin Lab Anal 2018;32:e22389. https://doi.org/10.1002/jcla.22389
Nishikawa H, Nakatsuka Y, Shiba M, et al. Increased plasma galectin-3 preceding the development of delayed cerebral infarction and eventual poor outcome in non-severe aneurysmal subarachnoid hemorrhage. Transl Stroke Res 2018;9:110–119. https://doi.org/10.1007/s12975-017-0564-0
Nakatsuka Y, Shiba M, Nishikawa H, et al. Acute-phase plasma osteopontin as an independent predictor for poor outcome after aneurysmal subarachnoid hemorrhage. Mol Neurobiol 2018;55:6841–6849. https://doi.org/10.1007/s12035-018-0893-3
Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery 2006;59:21–27. https://doi.org/10.1227/01.neu.0000218821.34014.1b
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–458. https://doi.org/10.1038/bmt.2012.244
Liu AY, Zheng H, Ouyang G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol 2014;37:150–156. https://doi.org/10.1016/j.matbio.2014.04.007
Matsusaka M, Kabata H, Fukunaga K, et al. Phenotype of asthma related with high serum periostin levels. Allergol Int 2015;64:175–180. https://doi.org/10.1016/j.alit.2014.07.003
Satirapoj B, Wang Y, Chamberlin MP, et al. Periostin: novel tissue and urinary biomarker of progressive renal injury induces a coordinated mesenchymal phenotype in tubular cells. Nephrol Dial Transplant 2012;27:2702–2711. https://doi.org/10.1093/ndt/gfr670
Bobolea I, Barranco P, Del Pozo V, et al. Sputum periostin in patients with different severe asthma phenotypes. Allergy 2015;70:540–546. https://doi.org/10.1111/all.12580
Fujishima H, Okada N, Matsumoto K, et al. The usefulness of measuring tear periostin for the diagnosis and management of ocular allergic diseases. J Allergy Clin Immunol 2016;138:459–467. https://doi.org/10.1016/j.jaci.2015.11.039
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010;37:13–25. https://doi.org/10.1016/j.nbd.2009.07.030
Sabri M, Lass E, Macdonald RL. Early brain injury: a common mechanism in subarachnoid hemorrhage and global cerebral ischemia. Stroke Res Treat 2013;2013:394036. https://doi.org/10.1155/2013/394036
Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol 2018;9:754. https://doi.org/10.3389/fimmu.2018.00754
Liu L, Fujimoto M, Nakano F, et al. Deficiency of tenascin-C alleviates neuronal apoptosis and neuroinflammation after experimental subarachnoid hemorrhage in mice. Mol Neurobiol 2018;55:8346–8354. https://doi.org/10.1007/s12035-018-1006-z
Okada T, Kawakita F, Nishikawa H, et al. Selective Toll-like receptor 4 antagonists prevent acute blood-brain barrier disruption after subarachnoid hemorrhage in mice. Mol Neurobiol 2018. https://doi.org/10.1007/s12035-018-1145-2
Kawakita F, Fujimoto M, Liu L, et al. Effects of Toll-like receptor 4 antagonists against cerebral vasospasm after experimental subarachnoid hemorrhage in mice. Mol Neurobiol 2017;54:6624–6633. https://doi.org/10.1007/s12035-016-0178-7
Fujimoto M, Shiba M, Kawakita F, et al. Effects of tenascin-C knockout on cerebral vasospasm after experimental subarachnoid hemorrhage in mice. Mol Neurobiol 2018;55:1951–1958. https://doi.org/10.1007/s12035-017-0466-x
Fujimoto M, Shiba M, Kawakita F, et al. Deficiency of tenascin-C and attenuation of blood-brain barrier disruption following experimental subarachnoid hemorrhage in mice. J Neurosurg 2016;124:1693–1702. https://doi.org/10.3171/2015.4.jns15484
Pang J, Chen Y, Kuai L, et al. Inhibition of blood-brain barrier disruption by an apolipoprotein E-mimetic peptide ameliorates early brain injury in experimental subarachnoid hemorrhage. Transl Stroke Res 2017;8:257–272. https://doi.org/10.1007/s12975-016-0507-1
Hoersch S, Andrade-Navarro MA. Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evol Biol 2010;10:30. https://doi.org/10.1186/1471-2148-10-30
Nishikawa H, Suzuki H. Implications of periostin in the development of subarachnoid hemorrhage-induced brain injuries. Neural Regen Res 2017;12:1982–1984. https://doi.org/10.4103/1673-5374.221150
Shimamura M, Taniyama Y, Katsuragi N, et al. Role of central nervous system periostin in cerebral ischemia. Stroke 2012;43:1108–1114. https://doi.org/10.1161/strokeaha.111.636662
Acknowledgments
We thank Ms. Chiduru Nakamura (Department of Neurosurgery, Mie University Graduate School of Medicine) for her technical assistance. This work was funded by a grant-in-aid for Scientific Research from Japan Society for the Promotion of Science (grant number: 17K10825) to Dr. Suzuki.
Members along with their affiliations listed in Supplementary Material (Appendix).
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The Institutional Ethics Committee approved the study, and written informed consent was obtained from the relatives.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kanamaru, H., Kawakita, F., Nakano, F. et al. Plasma Periostin and Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. Neurotherapeutics 16, 480–490 (2019). https://doi.org/10.1007/s13311-018-00707-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-018-00707-y