Abstract
Here, we review the role of pituitary adenylate cyclase-activating peptide-38 (PACAP38) in migraine pathophysiology and data implicating PAC1 receptor as a future drug target in migraine. Much remains to be fully elucidated about migraine pathophysiology, but recent attention has focused on signaling molecule PACAP38, a vasodilator able to induce migraine attacks in patients who experience migraine without aura. PACAP38, with marked and sustained effect, dilates extracerebral arteries but not the middle cerebral artery. The selective affinity of PACAP38 to the PAC1 receptor makes this receptor a highly interesting and potential novel target for migraine treatment. Efficacy of antagonism of this receptor should be investigated in randomized clinical trials.
Similar content being viewed by others
Introduction
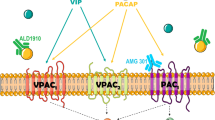
Since its discovery in 1989 [1], pituitary adenylate cyclase-activating peptide (PACAP) has gained considerable interest as a key molecule in migraine [2,3,4]; importantly, more recently, one of its receptors has been implicated as a treatment target for this debilitating neurological disease [5]. PACAP hails from the same superfamily as structurally related vasoactive intestinal peptide (VIP) [6]. Two bioactive forms exist: a 38-amino acid peptide (PACAP38) and a 27-amino acid peptide (PACAP27) [1]; the former accounting for 90% of mammalian tissue PACAP. In the nervous system, PACAP38 acts as a hormone, a neurotransmitter, and a neuromodulator [6, 7]. PACAP38 crosses the blood–brain barrier (BBB) via a protein transport system, which is responsible for transport in both directions, that is, from blood to brain and from brain to blood. While PACAP38 transport over the BBB occurs in a saturable manner, PACAP27 influx is nonsaturable. Although, both PACAPs cross the BBB, their effect on the central nervous system is questionable owing to 1) rapid efflux back to the blood from brain, and 2) degradation of the peptide [8]. PACAP38 is found in several important structures of interest in migraine pathophysiology—notably in sensory and parasympathetic perivascular fibers [9, 10], the trigeminal [11] and sphenopalatine [12] ganglia, and in the trigeminal nucleus caudalis (TNC) (see Fig. 1) [34]. In mast cells in human skin, PACAP38 causes degranulation and histamine release [35]. PACAP38 exerts its effects through at least 3 different receptors—the VPAC1, VPAC2, and PAC1 receptors [36], which are all G protein-coupled receptors that increase intracellular cyclic adenosine monophosphate [37]. PACAP38 shares affinity for the VPAC1–2 receptors with the structurally similar parasympathetic signaling molecule VIP [36], but surpasses VIP 300 to 1000-fold in affinity for the PAC1 receptor. mRNA of these receptors is found in trigeminal (sensory) and otic (parasympathetic) ganglia, with only VPAC1 found in sphenopalatine ganglia [38], and all 3 receptors are found in cerebral and cranial vessels (see Fig. 2) [38, 48]. Vasodilation and mast-cell degranulation are mediated by VPAC1–2 receptors. The PAC1 receptor is involved in multiple physiological functions [49], including chronic pain.
Schematic illustration of distribution of pituitary adenylate cyclase activating peptide (PACAP) in cingulate cortex [13], frontal cortex [13], hypothalamus [14,15,16,17,18,19,20], trigeminal ganglion (trig ganglion) [21,22,23], superior cervical ganglion (sup cerv ganglion) [13], thalamus [15, 24,25,26,27], occipital cortex [28], periaqueductal grey (PAG) [29], cerebellum [13, 19, 25, 30, 31], and pontine nuclei [27, 32, 33]
Schematic illustration of distribution of pituitary adenylate cyclase activating peptide (PACAP) receptors PAC1, VPAC1, and VPAC2 in cingulate cortex [39, 40], frontal cortex [40], hypothalamus [39,40,41], trigeminal ganglion (trig ganglion) [42], superior cervical ganglion (sup cerv ganglion) [43, 44], thalamus [39,40,41], cerebellum [39, 40, 45, 46], pontine nuclei [39, 40], and cranial arteries[47]
Here, we review studies implicating PACAP38 in migraine pathophysiology and discuss data sparking interest in the PAC1 receptor as a future drug target in migraine.
PACAP38 Provocation Studies
The continued development of human experimental models of migraine have helped elucidate migraine pathophysiology [50]. Recent advances include combining provocation studies with advanced imaging techniques such as structural and functional magnetic resonance imaging [51, 52].
A first systematic study investigated the effect of PACAP38 on cerebral hemodynamics in healthy volunteers and reported no effect on regional cerebral blood flow (when corrected for PACAP38-induced hypocapnia) [53]. Mild headache was noted as a side effect of infusion of PACAP38 at doses of 10 pmol/kg/min in 10 of 12 patients (83%), as well as a heart rate increased by 40% to 50%. Owing to the latter and other dose-dependent changes in vital signs a maximum dose of 10 pmol/kg/min was chosen for future provocation studies—a finding that was later upheld after investigation of headache inducing abilities of PACAP38 at lower doses [54].
The first study specifically investigating the headache and migraine-inducing abilities of PACAP38 elucidated responses in 12 healthy volunteers and 12 patients with migraine without aura (MO) [2]. In both groups, participants completed a randomized, double-blind, placebo-controlled crossover study comparing 20-min intravenous infusion of PACAP38 (10 pmol/kg/min over 20 min) to saline. Healthy volunteers reported 100% incidence of headache after PACAP38 and, interestingly, 2 of 12 reported migraine-like attack within 1 h of PACAP38 infusion. In patients with MO, 58% reported migraine attacks after PACAP38 versus none after placebo. In the same study a modest dilation of the middle cerebral artery (MCA) by 9.5% was calculated based on velocity measurements in the MCA and the assumption that cerebral blood flow was unchanged [53]. Moreover, the superficial temporal artery (STA) was dilated by 37.5%, which lasted throughout the 90-min observation period. Interestingly, migraine attacks occurred at a mean time of 6 h after the start of the infusion, suggesting the link between sustained vasodilation and PACAP38-induced delayed migraine attacks. To explore this a double-blind, placebo-controlled study investigated vascular responses after PACAP38 and placebo in 14 healthy volunteers by high-resolution magnetic resonance angiography (MRA) [55]. The major outcome was that PACAP38 dilated the middle meningeal artery (MMA; extracranial segment of the artery) over the 5-h observation period. In contrast, PACAP38 did not dilate MCA (intracerebral artery). Furthermore, migraine abortive treatment (sumatriptan injection 6 mg) had no effect on the MCA but reversed the MMA dilation and reduced the headache accompanying dilation.
To further elucidate mechanisms underlying PACAP38-induced migraine, a double-blind, randomized, crossover study in 22 patients with MO examined vascular responses after 20-min infusion of PACAP38 and VIP by MRA [52]. This study demonstrated that migraine induction was higher after PACAP38 (73% of patients) than with VIP (18% of patients). Both peptides dilated the STA and MMA but not the MCA, which is in contrast to findings of MCA increase and no change in STA and MMA in spontaneous migraine attacks in patients with migraine [56]. The discrepancy between arterial circumference during spontaneous and PACAP-induced migraine attack may be caused by the difference in time. In PACAP38-induced migraine experiments, patients underwent MRA scans at a very early time point, when PACAP38 had a marked dilatory effect on the extracranial arteries. During spontaneous attacks patients were scanned many hours into the attacks. Therefore, direct comparison of arterial circumference during provoked and spontaneous migraine is not possible. Interestingly, MMA dilation returned to baseline 2 h after start of VIP infusion but sustained (> 2 h) following PACAP38. PACAP38 receptors are found in both human meningeal and cerebral vessels [38, 48]. The vasodilator effect of PACAP38 is suggested to be mediated via the VPAC1–2 receptors, which are shared with VIP [48, 57, 58]. Although in vivo rat studies have yielded contradictory results reporting the VPAC1–2 receptors as mainly responsible for the PACAP38-induced meningeal vasodilation [57, 58] neither of the two in vivo studies reported any significant inhibition of this vasodilation by a PAC1 receptor antagonist. Previous studies pointed out the importance of mast-cell degranulation and histamine release in PACAP38-induced prolonged vasodilation [59, 60]. However, a human provocation study found no increase in serum tryptase, a marker for mast-cell degranulation, after PACAP38 [52]. It is important to note that possible mast-cell degranulation localized in dura might not adequately be reflected in antecubital vein blood.
To investigate the differential impact of PACAP38 and VIP on intrinsic functional brain connectivity, one study employed resting-state functional magnetic resonance imaging, a method analyzing the functional connectivity between the various parts of the brain [51]. PACAP38, but not VIP, was associated with altered connectivity in the three networks investigated. These networks, the salience, sensorimotor, and default network, have been implicated in cognition, emotional processing [61,62,63,64], photo- and phonophobia [65], and pain processing [66]. Whether the reported alterations are specific for PACAP38-induced migraine attacks is unknown. A similar study in other pain conditions than migraine may further elucidate this.
To investigate a possible genetic component in susceptibility to migraine after PACAP38, a double-blind human provocation study [67] compared PACAP38 migraine induction in patients with high (≥2 first-degree relatives with MO) and low (≤1 first-degree relative with MO) family load of migraine. In addition, based on previous genotyping, patients were stratified on presence of 2 or 0 risk alleles of a single nucleotide polymorphism, rs2274316, known as a risk factor in migraine and located within the MEF2D gene, which is involved in regulating PACAP expression [68]. Results showed no difference in migraine induction between groups—either based on family load or allele status. Thus, no apparent genetic susceptibility to PACAP38 was found.
The PAC1 Receptor and Future Directions
Several studies have shed light on the fluctuations of PACAP38 in ictal and interictal phases of migraine and in comparison to healthy volunteers. While PACAP38 levels in patients with migraine are higher ictally than interictally [69, 70], these increases are actually not statistically higher than PACAP38 levels in healthy controls [69]. Furthermore, conflicting results on interictal PACAP38 levels in patients with migraine have been reported [71]. Two studies reported lower PACAP levels in patients with migraine interictally than in healthy controls [69, 72], whereas one recent study reports comparable PACAP levels interictally in patients with migraine and controls [73]. This discrepancy can be explained by interassay differences. Interictally lower PACAP levels in patients with migraine could indicate chronic depletion of PACAP38 in the trigeminovascular system caused by repeated attack activity [74]. In an animal model, Han et al. [74] established a model of chronic migraine by repeated dural exposure to an inflammatory soup. In both plasma and trigeminal ganglion (TG), rats subsequently showed decreased PACAP38 levels. Interestingly, increased PAC1 receptor mRNA expression in TG but not TNC was reported. The mRNA expression of VPAC1–2 receptors, which PACAP38 and VIP share equal affinity for, was not significantly increased in TG and TNC [74]. Given that VIP did not induce migraine [75] and that PAC1 receptor mRNA was expressed in meningeal arteries [48], TG neurons [38], and TNC [34], the PAC1 receptor should be considered as a viable candidate for targeted migraine treatment. Interestingly, one in vitro study reported that administration of the selective PAC1 receptor agonist maxadilan and the PAC1 receptor antagonist M65 on TG neurons increased intracellular free calcium concentration [76]. The authors suggested that the PAC1 receptor antagonist may also act as agonists on primary sensory neurons and that unknown receptors or splice variants linked to distinct signal transduction pathways might explain this effect [76].
PACAP38 signaling in migraine could be blocked in a number of ways. Small molecules or monoclonal antibodies could target the PAC1 receptor. Alternatively, targeting PACAP38 itself with monoclonal antibodies would be an option [77]. These strategies also hail from emerging results of targeting similar peptide calcitonin gene-related peptide (CGRP) in large-scale clinical trials showing promising results in migraine prevention [78,79,80]. At present, it is difficult to speculate on the possible mechanism of the treatment of monoclonal antibodies against PACAP itself or PAC1 receptor. Anti-CGRP antibody trials revealed no difference between compounds developed against CGRP or its receptor [78,79,80], and the exact site of action for anti-CGRP agents remains to be fully elucidated [81]. Whether targeting the PACAP38 signaling pathway will be efficacious in migraine treatment is unknown. Future phase II trials investigating this are currently planned [82, 83].
References
Miyata A, Jiang L, Dahl RD, et al. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun 1990; 170: 643–648.
Schytz HW, Birk S, Wienecke T, et al. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 2009; 132: 16–25.
Schytz HW, Schoonman GG, Ashina M. What have we learnt from triggering migraine? Curr Opin Neurol 2010; 23: 259–65.
Kaiser E a., Russo AF. CGRP and migraine: Could PACAP play a role too? Neuropeptides 2013; 47: 451–461.
Market Realist. Behind Amgen's plans to penetrate the migraine segment. Available at: http://marketrealist.com/2016/05/amgen-plans-penetrate-migraine-segment-multiple-investigational-drugs/. Accessed December 19, 2017.
Vaudry D, Gonzalez BJ, Basille M, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 2000; 52: 269–324.
Vécsei L, Tuka B, Tajti J. Role of PACAP in migraine headaches. Brain 2014; 137: 650–651.
Banks WA, Kastin A, Komaki G, et al. Passage of pituary adenylate cyclase activating polypeptide1-27 and pituary adenylate cyclase activating polypeptide1-38 across the blood-brain barrier. J Pharmacol Exp Ther 1993; 267: 690–696.
Gulbenkian S, Uddman R, Edvinsson L. Neuronal messengers in the human cerebral circulation. Peptides 2001; 22: 995–1007.
Edvinsson L, Uddman R. Neurobiology in primary headaches. Brain Res Rev 2005; 48: 438–456.
Tajti J, Uddman R, Moller S, et al. Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Auton Nerv Syst 1999; 76: 176–183.
Uddman R, Tajti J, Moller S, et al. Neuronal messengers and peptide receptors in the human sphenopalatine and otic ganglia. Brain Res 1999; 826: 193–199.
Mikkelsen JD, Hannibal J, Larsen PJ, et al. Pituitary adenylate cyclase activating peptide (PACAP) mRNA in the rat neocortex. Neurosci Lett 1994; 171: 121–124.
Koves K, Arimura A, Somogyvari-Vigh A, et al. Immunohistochemical demonstration of a novel hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide, in the ovine hypothalamus. Endocrinology 1990; 127: 264–271.
Vigh S, Arimura A, Koves K, et al. Immunohistochemical localization of the neuropeptide, pituitary adenylate cyclase activating polypeptide (PACAP), in human and primate hypothalamus. Peptides 1991; 12: 313–318.
Kivipelto L, Absood A, Arimura A, et al. The distribution of pituitary adenylate cyclase-activating polypeptide-like immunoreactivity is distinct from helodermin- and helospectin-like immunoreactivities in the rat brain. J Chem Neuroanat 1992; 5: 85–94.
Tamada Y, Tanaka M, Ichitani Y, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP)-like immunoreactive neuronal elements in rat hypothalamus and median eminence with special reference to morphological background of its effect on anterior pituitary--light and electron microscopic. Neurosci Lett 1994; 180: 105–108.
Hannibal J, Mikkelsen JD, Fahrenkrug J, et al. Pituitary adenylate cyclase-activating peptide gene expression in corticotropin-releasing factor-containing parvicellular neurons of the rat hypothalamic paraventricular nucleus is induced by colchicine, but not by adrenalectomy, acute osmotic, ether, or restraint stress. Endocrinology 1995; 136: 4116–4124.
Hannibal J, Mikkelsen JD, Clausen H, et al. Gene expression of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat hypothalamus. Regul Pept 1995; 55: 133–148.
Mikkelsen JD, Hannibal J, Fahrenkrug J, et al. Pituitary adenylate cyclase activating peptide-38 (PACAP-38), PACAP-27, and PACAP related peptide (PRP) in the rat median eminence and pituitary. J Neuroendocrinol 1995; 7: 47–55.
Moller K, Zhang YZ, Hakanson R, et al. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience 1993; 57: 725–732.
Mulder H, Uddman R, Moller K, et al. Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience 1994; 63: 307–312.
Dun NJ, Miyazaki T, Tang H, et al. Pituitary adenylate cyclase activating polypeptide immunoreactivity in the rat spinal cord and medulla: implication of sensory and autonomic functions. Neuroscience 1996; 73: 677–686.
Masuo Y, Suzuki N, Matsumoto H, et al. Regional distribution of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay. Brain Res 1993; 602: 57–63.
Takahashi K, Totsune K, Murakami O, et al. Pituitary adenylate cyclase activating polypeptide (PACAP)-like immunoreactivity in human hypothalamus: co-localization with arginine vasopressin. Regul Pept 1994; 50: 267–275.
Palkovits M, Somogyvari-Vigh A, Arimura A. Concentrations of pituitary adenylate cyclase activating polypeptide (PACAP) in human brain nuclei. Brain Res 1995; 699: 116–120.
Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol 2002; 453: 389–417.
Nonaka N, Farr SA, Nakamachi T, et al. Intranasal administration of PACAP: uptake by brain and regional brain targeting with cyclodextrins. Peptides 2012; 36: 168–175.
Das M, Vihlen CS, Legradi G. Hypothalamic and brainstem sources of pituitary adenylate cyclase-activating polypeptide nerve fibers innervating the hypothalamic paraventricular nucleus in the rat. J Comp Neurol 2007; 500: 761–776.
Ghatei M a, Takahashi K, Suzuki Y, et al. Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues. J Endocrinol 1993; 136: 159–166.
Nielsen HS, Hannibal J, Fahrenkrug J. Expression of pituitary adenylate cyclase activating polypeptide (PACAP) in the postnatal and adult rat cerebellar cortex. Neuroreport 1998; 9: 2639–2642.
Tajti J, Uddman R, Edvinsson L. Neuropeptide localization in the ‘migraine generator’ region of the human brainstem. Cephalalgia 2001; 21: 96–101.
Farnham MMJ, Li Q, Goodchild AK, et al. PACAP is expressed in sympathoexcitatory bulbospinal C1 neurons of the brain stem and increases sympathetic nerve activity in vivo. Am J Physiol Regul Integr Comp Physiol 2008; 294: R1304-R1311.
Uddman R, Tajti J, Hou M, et al. Neuropeptide expression in the human trigeminal nucleus caudalis and in the cervical spinal cord C1 and C2. Cephalalgia; 22: 112–116.
Ødum L, Petersen LJ, Skov PS, et al. Pituitary adenylate cyclase activating polypeptide (PACAP) is localized in human dermal neurons and causes histamine release from skin mast cells. Inflamm Res 1998; 47: 488–492.
Harmar AJ, Fahrenkrug J, Gozes I, et al. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol 2012; 166: 4–17.
Dickson L, Finlayson K. Pharmacology & Therapeutics VPAC and PAC receptors: from ligands to function. Pharmacol Ther 2009; 121: 294–316.
Knutsson M, Edvinsson L. Distribution of mRNA for VIP and PACAP receptors in human cerebral arteries and cranial ganglia. Neuroreport 2002; 13: 507–509.
Hashimoto H, Nogi H, Mori K, et al. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol 1996; 371: 567–577.
Shioda S, Shuto Y, Somogyvari-Vigh A, et al. Localization and gene expression of the receptor for pituitary adenylate cyclase-activating polypeptide in the rat brain. Neurosci Res 1997; 28: 345–354.
Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology 1994; 135: 2662–2680.
Chaudhary P, Baumann TK. Expression of VPAC2 receptor and PAC1 receptor splice variants in the trigeminal ganglion of the adult rat. Brain Res Mol Brain Res 2002; 104: 137–142.
Nogi H, Hashimoto H, Hagihara N, et al. Distribution of mRNAs for pituitary adenylate cyclase-activating polypeptide (PACAP), PACAP receptor, vasoactive intestinal polypeptide (VIP), and VIP receptors in the rat superior cervical ganglion. Neurosci Lett 1997; 227: 37–40.
Brandenburg CA, May V, Braas KM. Identification of endogenous sympathetic neuron pituitary adenylate cyclase-activating polypeptide (PACAP): depolarization regulates production and secretion through induction of multiple propeptide transcripts. J Neurosci 1997; 17: 4045–4055.
Zhou CJ, Kikuyama S, Shibanuma M, et al. Cellular distribution of the splice variants of the receptor for pituitary adenylate cyclase-activating polypeptide (PAC(1)-R) in the rat brain by in situ RT-PCR. Brain Res Mol Brain Res 2000; 75: 150–158.
Basille M, Vaudry D, Coulouarn Y, et al. Comparative distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) binding sites and PACAP receptor mRNAs in the rat brain during development. J Comp Neurol 2000; 425: 495–509.
Baun M, Hay-Schmidt A, Edvinsson L, et al. Pharmacological characterization and expression of VIP and PACAP receptors in isolated cranial arteries of the rat. Eur J Pharmacol 2011; 670: 186–194.
Chan KY, Baun M, De Vries R, et al. Pharmacological characterization of VIP and PACAP receptors in the human meningeal and coronary artery. Cephalalgia 2011; 31: 181–9.
May V, Parsons RL. G protein-coupled receptor endosomal signaling and regulation of neuronal excitability and stress responses: signaling options and lessons from the PAC1 receptor. J Cell Physiol 2017; 232: 698–706.
Ashina M, Hansen JM, Olesen J. Pearls and pitfalls in human pharmacological models of migraine: 30 years’ experience. Cephalalgia 2013; 33: 540–553.
Amin FM, Hougaard A, Magon S, et al. Change in brain network connectivity during PACAP38-induced migraine attacks: a resting-state functional MRI study. Neurology 2016; 86: 180–187.
Amin FM, Hougaard A, Schytz HW, et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 2014; 137: 779–794.
Birk S, Sitarz JT, Petersen KA, et al. The effect of intravenous PACAP38 on cerebral hemodynamics in healthy volunteers. Regul Pept 2007; 140: 185–191.
Vollesen ALH, Guo S, Ashina M. PACAP38 dose-response pilot study in migraine patients. Cephalalgia 2016; 0: 1–5.
Amin FM, Asghar MS, Guo S, et al. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia 2012; 32: 140–149.
Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol 2013; 12: 454–461.
Boni L, Ploug K, Olesen J, et al. The in vivo effect of VIP, PACAP-38 and PACAP-27 and mRNA expression of their receptors in rat middle meningeal artery. Cephalalgia 2009; 29: 837–847.
Akerman S, Goadsby PJ. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: relevance to migraine. Sci Transl Med 2015; 7: 308ra157.
Baun M, Pedersen MHF, Olesen J, et al. Dural mast cell degranulation is a putative mechanism for headache induced by PACAP-38. Cephalalgia 2012; 32: 337–45.
Bhatt DK, Gupta S, Olesen J, et al. PACAP-38 infusion causes sustained vasodilation of the middle meningeal artery in the rat: possible involvement of mast cells. Cephalalgia 2014; 34: 877–886.
Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27: 2349–2356.
Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008; 1124: 1–38.
Farmer MA, Baliki MN, Apkarian AV. A dynamic network perspective of chronic pain. Neurosci Lett 2012; 520: 197–203.
Lindquist KA, Wager TD, Kober H, et al. The brain basis of emotion: a meta-analytic review. Behav Brain Sci 2012; 35: 121–143.
Sava SL, de Pasqua V, Magis D, et al. Effects of visual cortex activation on the nociceptive blink reflex in healthy subjects. PLOS ONE 2014; 9: e100198.
Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. a review and meta-analysis (2000). Neurophysiol Clin Neurophysiol 2000; 30: 263–288.
Guo S, Vollesen ALH, Hansen RD, et al. Part I: pituitary adenylate cyclase-activating polypeptide-38 induced migraine-like attacks in patients with and without familial aggregation of migraine. Cephalalgia 2017; 37: 125–135.
Flavell SW, Kim TK, Gray JM, et al. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron 2008; 60: 1022–1038.
Tuka B, Helyes Z, Markovics A, et al. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 2013; 33: 1085–95.
Zagami AS, Edvinsson L, Goadsby PJ. Pituitary adenylate cyclase activating polypeptide and migraine. Ann Clin Transl Neurol 2014; 1(12): 1036–40.
Riesco N, Cernuda-Morollón E, Pascual J. Neuropeptides as a marker for chronic headache. Curr Pain Headache Rep 2017;21:18.
Han X, Dong Z, Hou L, et al. Interictal plasma pituitary adenylate cyclase-activating polypeptide levels are decreased in migraineurs but remain unchanged in patients with tension-type headache. Clin Chim Acta 2015; 450: 151–154.
Cernuda-Morollón E, Riesco N, Martínez-Camblor P, et al. No change in interictal PACAP Levels in peripheral blood in women with chronic migraine. Headache 2016; 56: 1448–1454.
Han X, Ran Y, Su M, et al. Chronic changes in pituitary adenylate cyclase-activating polypeptide and related receptors in response to repeated chemical dural stimulation in rats. Mol Pain 2017; 13:1–10.
Rahmann A, Wienecke T, Hansen JM, et al. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia 2008; 28: 226–236.
Sághy E, Payrits M, Helyes ZS, et al. Stimulatory effect of pituitary adenylate cyclase-activating polypeptide 6-38, M65 and vasoactive intestinal polypeptide 6-28 on trigeminal sensory neurons. Neuroscience 2015; 308: 144–156.
Jansen-Olesen I, Baun M, Amrutkar D V, et al. PACAP-38 but not VIP induces release of CGRP from trigeminal nucleus caudalis via a receptor distinct from the PAC1 receptor. Neuropeptides 2014; 48: 53–64.
Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15: 382–390.
Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015; 14: 1091–1100.
Dodick DW, Goadsby PJ, Spierings ELH, et al. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 2014; 13: 885–892.
Edvinsson L. The trigeminovascular pathway: role of CGRP and CGRP receptors in migraine. Headache J Head Face Pain 2017; 57: 47–55.
Patrick M. Behind Amgen’s plans to penetrate the migraine segment. 2016; Available at: http://marketrealist.com/2016/05/amgen-plans-penetrate-migraine-segment-multiple-investigational-drugs/. Accessed February 27, 2017.
Alder Biopharmaceuticals. Robust development pipeline. 2017; Available at: http://www.alderbio.com/therapeutics/pipeline/. Accessed February 27, 2017).
Acknowledgements
The study was supported by grants from Lundbeck Foundation (R155-2014-171) and Research Foundation of Rigshospitalet. Messoud Ashina is a consultant, speaker or scientific advisor for Allergan, Amgen, Alder, ATI, Eli Lilly, Novartis, and Teva; primary investigator for Amgen 20120178 (phase II), 20120295 (phase II), 20130255 (OLE), 20120297 (phase III), Alder ALD403-CLIN-001 (phase III), Amgen PAC1 20150308 (phase IIa), and GM-11 gamma-Core-R trials; and reports a grant from Lundbeck Foundation (R155-2014-171). Anne Luise Haulund Vollesen has no conflicts of interest. Faisal Amin reports personal fees from Novartis and grants from Allergan outside the submitted work.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
Required Author Forms Disclosure forms provided by the authors are available with the online version of this article. (PDF 1225 kb)
Rights and permissions
About this article
Cite this article
Vollesen, A.L.H., Amin, F.M. & Ashina, M. Targeted Pituitary Adenylate Cyclase-Activating Peptide Therapies for Migraine. Neurotherapeutics 15, 371–376 (2018). https://doi.org/10.1007/s13311-017-0596-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-017-0596-x