Abstract
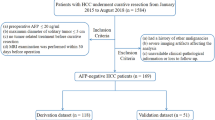
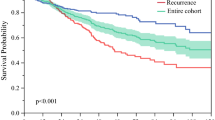
To validate a previously reported alpha-fetoprotein (AFP) model (including three variables: preoperative image-diagnosed tumor number and size and AFP level) for the prediction of recurrence in hepatocellular carcinoma (HCC) patients who have undergone liver resection (LR). This retrospective study enrolled patients who underwent curative LR for newly diagnosed HCC in our institution between 2011 and 2018. The probabilities of overall survival (OS) and recurrence were compared according to the aforementioned AFP model. A total of 838 patients were included. AFP score ≥ 3 versus ≤ 2 independently predicted recurrence and OS. However, net reclassification improvements (NRI) indicated that the AFP model was not superior to the Barcelona Clinic Liver Cancer (BCLC) system for predicting 1-year recurrence (p = 0.746). Relatedly, we developed a modified AFP model based on our cohort. The modified AFP score ≥ 3 versus ≤ 2 independently predicted recurrence and OS. However, NRI again indicated that the modified AFP model was not superior to the BCLC system for predicting 1-year recurrence (p = 0.69). Patients with a modified AFP score ≤ 2 had a risk of recurrence similar to that of patients with a modified AFP score ≥ 3 in BCLC stage 0-A (p = 0.57). However, patients with a modified AFP score ≤ 2 had a lower risk of recurrence than patients with a modified AFP score ≥ 3 in BCLC stage B-C (p = 0.02). The original AFP model was not feasible in our cohort. However, the modified AFP model may be useful for predicting recurrence in BCLC B-C patients who underwent LR in our cohort.


Similar content being viewed by others
References
EASL Clinical Practice Guidelines (2018) Management of hepatocellular carcinoma. J Hepatol 69:182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750. https://doi.org/10.1002/hep.29913
Tsilimigras DI, Bagante F, Moris D et al (2020) Recurrence patterns and outcomes after resection of hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer Criteria. Ann Surg Oncol 27:2321–2331. https://doi.org/10.1245/s10434-020-08452-3
Liu YW, Yong CC, Lin CC et al (2020) Liver resection of hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guideline recommendations: results from a high-volume liver surgery center in East Asia. J Surg Oncol 122:1587–1594. https://doi.org/10.1002/jso.26183
Torzilli G, Belghiti J, Kokudo N et al (2013) A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg 257:929–937. https://doi.org/10.1097/SLA.0b013e31828329b8
Ding HF, Zhang XF, Bagante F et al (2021) Prediction of tumor recurrence by a-fetoprotein model after curative resection for hepatocellular carcinoma. Eur J Surg Oncol 47(3 Pt B):660–666. https://doi.org/10.1016/j.ejso.2020.10.017
A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators (1998) Hepatology 28:751–755. doi: https://doi.org/10.1002/hep.510280322.
Duvoux C, RoudoteThoraval F, Decaens T et al (2012) Liver transplantation for hepatocellular carcinoma: a model including afetoprotein improves the performance of milan criteria. Gastroenterology 143:986-994e3. https://doi.org/10.1053/j.gastro.2012.05.052 (quiz e14-e15)
Edmonson H, Steiner P (1954) Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 7:462–503. https://doi.org/10.1002/1097-0142(195405)7:3%3c462::aid-cncr2820070308%3e3.0.co;2-e
Everhart JE, Wright EC, Goodman ZD et al (2010) Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 51:585–594. https://doi.org/10.1002/hep.23315
Abou-Alfa GK, Pawlik TM, Shindoh J, et al (2017) Liver. In: Amin MB (ed) AJCC cancer staging manual, 8th edn. AJCC, Chicago, p 287
American Joint Committee on Cancer (2010) Edge SB, Byrd DR, Compton CC, et al (eds) American Joint Committee on cancer staging manual, 7th edn. Springer, New York, p 175
Benson AB, Abrams TA, Ben-Josef E et al (2009) NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 7:350–391. https://doi.org/10.6004/jnccn.2009.0027
European Association for the Study of the Liver, European Organisation For Research and Treatment of Cancer (2012) EASL-EORTC clinical practice guidelines. Management of hepatocellular carcinoma. J Hepatol 56:908–943. https://doi.org/10.1016/j.jhep.2011.12.001
Omata M, Lesmana LA, Tateishi R et al (2010) Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 4:439–474. https://doi.org/10.1007/s12072-010-9165-7
Bruix J, Sherman M; American Association for the Study of Liver Diseases (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
Mazzaferro V, Sposito C, Zhou J et al (2018) Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 154:128–139. https://doi.org/10.1053/j.gastro.2017.09.025
Omata M, Cheng AL, Kokudo N et al (2017) Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11:317–370. https://doi.org/10.1007/s12072-017-9799-9
Aggarwal A, Horwitz JK, Dolan D et al (2019) Hypo-vascular hepatocellular carcinoma and liver transplantation: Morphological characteristics and implications on outcomes. J Surg Oncol 120:1112–1118. https://doi.org/10.1002/jso.25700
Pelizzaro F, Cardin R, Penzo B et al (2021) Liquid biopsy in hepatocellular carcinoma: where are we now? Cancers (Basel) 13:2274. https://doi.org/10.3390/cancers13092274
Acknowledgements
The authors thank Cancer Center, Kaohsiung Chang Gung Memorial Hospital for the provision of HCC registry data. The authors thank Chih-Yun Lin and Nien-Tzu Hsu and the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work. The authors thank Dr. Toshiaki Nakano for the revision of the response to reviewers’ comments. This study was supported by Grant CMRPG8J1281 from the Chang Gung Memorial Hospital-Kaohsiung Medical Center, Taiwan.
Funding
This study was supported by Grant CMRPG8J1281 from the Chang Gung Memorial Hospital-Kaohsiung Medical Center, Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose for all authors.
Data availability
All data is available.
Ethics approval
The Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital approved this study (Reference number: 202000398B0) and waived the need for informed consent.
Informed consent
The IRB of our institution waived the need for informed consent due to this study is retrospective and observational in nature.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13304_2021_1147_MOESM1_ESM.pdf
Supplementary file1 A flowchart illustrating the inclusion/exclusion criteria can be found in Supplementary Fig. 1 (PDF 82 KB)
13304_2021_1147_MOESM2_ESM.tiff
Supplementary file2 ROC analysis identified that the optimal AFP score for stratifying patients as having low versus high risk of 1-year recurrence was a cut-off value of 2 (TIFF 719 KB)
13304_2021_1147_MOESM3_ESM.tiff
Supplementary file3 ROC analysis identified that the optimal modified AFP score for stratifying patients as having low versus high risk of 1-year recurrence was a cut-off value of 2 (TIFF 720 KB)
13304_2021_1147_MOESM4_ESM.tiff
Supplementary file4 Cumulative recurrence rate (a) and overall survival (b) after curative resection among patients with preoperative modified AFP score ≤2 versus patients with AFP score ≥3 (TIFF 151 KB)
13304_2021_1147_MOESM6_ESM.tiff
Supplementary file6 Cumulative recurrence rate after surgical resection among patients with modified AFP score ≤2 versus patients with AFP score ≥3 in subgroups of BCLC 0-A (a) and BCLC B-C (b) stage patients (TIFF 151 KB)
13304_2021_1147_MOESM8_ESM.tif
Supplementary file8 Cumulative recurrence rate after surgical resection among patients with modified AFP score ≤2 versus patients with AFP score ≥3 among patients with image-diagnosed macrovascular invasion (TIF 111 KB)
13304_2021_1147_MOESM9_ESM.tif
Supplementary file9 Cumulative recurrence rate after surgical resection among patients within and beyond the Metroticket 2.0 criteria (TIF 154 KB)
13304_2021_1147_MOESM10_ESM.tif
Supplementary file10 Cumulative recurrence rate after surgical resection among patients within and beyond the Metroticket 2.0 criteria in subgroups of BCLC 0-A stage patients (TIF 147 KB)
13304_2021_1147_MOESM11_ESM.tif
Supplementary file11 Cumulative recurrence rate after surgical resection among patients within and beyond the Metroticket 2.0 criteria in subgroups of BCLC B-C stage patients (TIF 146 KB)
Rights and permissions
About this article
Cite this article
Li, WF., Yen, YH., Liu, YW. et al. Validation of an alpha-fetoprotein model to predict recurrence after liver resection for hepatocellular carcinoma. Updates Surg 74, 1345–1352 (2022). https://doi.org/10.1007/s13304-021-01147-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-021-01147-8