Abstract
Objective
To investigate the effects of pentoxifylline (PTX) in combination with losartan compared to the high dose of losartan alone on serum markers of diabetic nephropathy such as HSP70, copeptin, CRP, and TNFα in patients with type 2 diabetes and nephropathy.
Methods
A single-center, randomized, double-blind, open-label clinical trial was conducted. Sixty-two patients were eligible and allocated to “PTX + losartan” and “high-dose losartan” arms of the trial using software for random number generation. The first arm received 400 mg PTX two times a day (BD) plus 50 mg losartan daily, while the second arm received 50 mg losartan two times a day (BD) for 12 weeks. Comparison of the biomarkers’ levels before and after treatment was done using paired sample t test variance. ANCOVA was applied to evaluate the comparative efficacy of the two interventions. The effect size was calculated and reported for each biomarker.
Results
Urine albumin excretion (UAE), hs-CRP, and HbA1c significantly decreased in both trial arms compared to the baseline measures. Copeptin and TNFα showed significant differences (after vs before) only in the losartan group (p = 0.017 and p = 0.043, respectively). The losartan arm was more successful in reducing TNFα, copeptin, HSP70, systolic blood pressure (SBP), and diastolic blood pressure (DBP) values (p = 0.045, effect size = 7.3%; p = 0.018, effect size 10.1%; p = 0.046, effect size 4.7%, p = 0.001, effect size 23%; p = 0.012, effect size 10.2%, respectively) and the PTX arm was associated with a superior reduction of UAE and hs-CRP levels (p = 0.018, effect size 9.1%; p = 0.028, effect size 9.2%, respectively).
Conclusion
Add-on PTX to losartan may have more effective anti-inflammatory and anti-albuminuric roles and therefore may be more applicable in the management of diabetic nephropathy compared with high-dose losartan alone.
Trail Registration
Trial number IRCT 20121104011356N10.
Similar content being viewed by others
Diabetic kidney disease (DKD), also known as diabetic nephropathy, is one of the severe causes of mortality and morbidity in patients with diabetes. |
Plenty of serum markers are reported to be associated with renal lesions such as circulating TNF receptors, serum cystatin C, CRP, TNFα, kidney injury molecule-1 (KIM-1), N-acetyl-beta-d-glucosaminidase (NAG), liver-type fatty acid-binding protein (L-FABP), heat shock protein 70 (HSP70), and copeptin. |
Pentoxifylline is an anti-inflammatory agent, which is a competitive nonselective phosphodiesterase inhibitor that raises intracellular cAMP, activates protein kinase A, inhibits TNF, and leukotriene, which may have effectiveness in chronic kidney disease. |
Patients in the pentoxifylline arm experienced comparatively superior reductions in serum hs-CRP levels and UAE rates, and patients in the losartan arm recorded larger reductions in HSP70, TNFα, copeptin, SBP, and DBP. |
Add-on pentoxifylline to losartan may be a more effective approach to reduce residual albuminuria and inflammation compared to high-dose losartan alone in the management of diabetic nephropathy. |
Introduction
Diabetic kidney disease (DKD), also known as diabetic nephropathy, is one of the severe causes of mortality and morbidity in patients with diabetes [1]. It is characterized by elevated urine albumin excretion (UAE) or decreased glomerular filtration rate (GFR) or both of these conditions. DKD arises in 20–40% of patients with diabetes [2]. Early detection of symptoms and effective management may slow down or even arrest the progression of DKD. Several risk factors have been established for DKD, including hyperglycemia, hypertension, age, sex, and duration of diabetes. Plenty of serum markers have been reported to be associated with renal lesions such as circulating tumor necrosis factor (TNF) receptors, serum cystatin C, CRP, TNFα [3], kidney injury molecule-1 (KIM-1) [4], N-acetyl-beta-d-glucosaminidase (NAG) [5], liver-type fatty acid-binding protein (L-FABP) [6, 7], heat shock protein 70 (HSP70) [8], and copeptin [9]. In addition, osteopontin and N-terminal pro-brain natriuretic peptide (NT-proBNP) are potential risk biomarkers for diabetic disease aggravation [10]. Vascular complications in diabetes are highly associated with inflammation, which is triggered by several factors such as obesity. Adipose tissue inflammation may lead to local hypoxia due to rapid expansion of adipose tissue without sufficient vascular adaptation [11]. The renin–angiotensin system also has a significant role in inflammation, insulin resistance, and vascular damage [12,13,14]. Pentoxifylline (PTX) is an anti-inflammatory agent, which is a competitive nonselective phosphodiesterase inhibitor that raises intracellular cAMP, activates protein kinase A, and inhibits TNF, and leukotriene [15,16,17].
There have been previous clinical trials with PTX which, despite their small sample size, have shown statistically significant effects of this drug on stabilizing plaques, slowing the progression of atherosclerosis, and decreasing the risk and improving the outcome of vascular events. Studies suggest that PTX exerts these effects by reducing inflammatory markers and improving blood flow [18, 19]. However, further studies are required to assess the scope of benefits conferred by PTX on outcomes of end-organ damage in patients with diabetes. Some trials showed the benefits of PTX combined with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) in the treatment of DKD [19,20,21,22,23,24,25,26,27]. We designed this trial to investigate the effects of PTX in combination with losartan compared to the high dose of losartan alone on markers of diabetic nephropathy such as HSP70, copeptin, hs-CRP, TNFα, and UAE in patients with type 2 diabetes and nephropathy.
Methods
Design
A single-center, randomized, double-blinded clinical trial was conducted. Patients were recruited through the diabetes clinic of Vali-Asr hospital (Tehran, Iran) from August 2019 to February 2020 (IRCT 20121104011356N10). The study protocol is available in the Iranian Registry of Clinical Trials (https://en.irct.ir/trial/46758) and use of human data was in accordance with guidelines of the Helsinki Declaration of 1964 and its later amendments. Written informed consent was obtained from each subject regarding the privacy and anonymity of data collection. All individuals’ experiments were approved by the National Institute for Medical Research Development (NIMAD) ethical committee (IR.NIMAD.REC.1398.193).
Subjects were found eligible if the following criteria were met: (1) diagnosis of type 2 diabetes mellitus based on American Diabetes Association (ADA 2019) criteria; (2) UAE rate at least 30 mg/24 h; (3) being on daily losartan 50 mg for at least 3 months. Exclusion criteria were (1) history of current infectious or malignant diseases, non-diabetic kidney disease including glomerulonephritis or tubulointerstitial diseases, retinal hemorrhage, acute myocardial infarction, or unstable angina; (2) history of cardiocerebrovascular or peripheral artery disease, and uncontrolled hypertension (i.e., systolic blood pressure [SBP] at least 140 mmHg and/or diastolic blood pressure [DBP] at least 90 mmHg); (3) history of anemia, hyperthyroidism, and hemodialysis; (4) baseline serum potassium concentration at least 5.5 meq/L; (5) estimated glomerular filtration rate (eGFR) less than 30 mL/min/1.73 m2; (6) pregnancy; and (7) PTX intolerance. The sample size calculation and power calculation were done on the basis of the proper sample size formula of statistical superiority design, and α and β were 0.05 and 0.2, respectively [28].
Sixty-two patients were eligible and allocated to PTX and losartan arms of the trial using software for random number generation. The first arm received 400 mg PTX two times a day (BD) and 50 mg losartan daily, while the second arm received 50 mg losartan two times a day (BD). Subjects were instructed regarding the side effects of the medication. After 12 weeks, subjects returned for a follow-up visit and were interviewed and examined using the same protocol as the baseline. Written informed consent was obtained from each subject regarding confidentiality and anonymity of data collected, but the details and purpose of the study were not disclosed. Tehran University of Medical Sciences’ board of ethics approved the study protocol.
Assessment
During the initial visit, patients were interviewed according to a pre-designed questionnaire and underwent a thorough physical examination afterward. Subjects were asked about the drug history for diabetes and hyperlipidemia. A standard sphygmomanometer (Riester, Big Ben adults, Germany) was used to measured blood pressure. Subjects were asked to rest in a sitting position for at least 10 min; two readings with 5-min intervals were averaged. Height was measured by employing standard stadiometer, with subjects standing; the nearest 0.1 cm was recorded. Weight was assessed via a digital scale (Beurer, GS49, Germany); hence, only light-weight clothing was permitted. The Quetelet formula used to calculate body mass index (BMI), using the of weight in kilograms divided by height squared in meters (kg/m2). The same examinations were performed at the 12-week follow-up visit.
Laboratory Evaluations
Subjects were instructed to fast overnight for at least 10 h at both initial and 12-week follow-up visits. The next morning, patients’ venous blood samples were drawn and stored at − 70 °C in the hospital laboratory. Fasting plasma glucose (FPG) concentrations were assessed by the enzymatic colorimetric method using the glucose oxidase (GOD) test. The percentage of glycated hemoglobin A1c (HbA1c) was determined using high-performance liquid chromatography (HPLC). Enzymatic methods (Pars Azmun commercial kits, Karaj, Iran) were employed to measure serum concentrations of total cholesterol, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and triglycerides (TG). Urine albumin concentrations were quantified by an immunoturbidimetric assay. Serum hs-CRP levels were quantitatively assessed by commercial kits (DRG kit, Germany) using the ELISA (enzyme-linked immunosorbent assay) method. Serum HSP70 levels were assessed using the ELISA method (ELISA kit, Bioassay Technology Laboratory China) with a sensitivity of 0.12 ng/mL (assay range 0.3–90 ng/mL) and interassay and intra-assay coefficient of variations of less than 8% and less than 10%, respectively. TNFα levels were measured using the ELISA method (ELISA kit, Bioassay Technology Laboratory China) with a sensitivity of 1.52 ng/mL (assay range 3–900 ng/mL) and interassay and intra-assay coefficient of variations of less than 8% and less than 10%, respectively. Copeptin levels were measured using the ELISA method (ELISA kit, Bioassay Technology Laboratory China) with a sensitivity of 0.024 ng/mL (standard range 0.05–20 ng/mL) and interassay and intra-assay coefficient of variations of less than 8% and less than 10%, respectively.
Statistical Analysis
To test the normality of our study population Kolmogorov–Smirnov and Shapiro–Wilk normality tests were performed and P–P plot and histogram were illustrated. The null hypothesis was rejected for all the variables; thus, they were normal. T test and chi-square analysis were performed to assess the demographic and laboratory data of the group of patients on PTX and losartan and the group of patients on high-dose losartan. Data were reported as mean ± standard deviation (SD) for continuous variables and as proportions for categorical variables. To assess the difference between levels of biomarkers (i.e., HSP70, copeptin, CRP, and TNFα) before and after the treatment in each group of patients, paired sample t test was used. Univariate analysis of covariance (ANCOVA) was applied to evaluate the comparative efficacy of the two interventions. The measured markers were entered as dependent variables, while the possible confounder categories and baseline measurements were treated as covariates. The independent variable was the intervention categories.
Model 1 was adjusted for baseline measurements. Model 2 controlled for baseline measurements, age, and gender. Model 3 controlled for baseline measurements, age, gender, BMI, and eGFR. Model 4 controlled for baseline measurements, age, gender, BMI, eGFR, SBP, and DBP.
The effect size was calculated from partial eta squared. The values of 1%, 6%, and 13.8% indicate small, medium, and large effect sizes, respectively. The statistically significant level was set at p < 0.05 for all tests. Interaction plots were illustrated. SPSS software version 25 for Windows was used for the statistical analysis.
Results
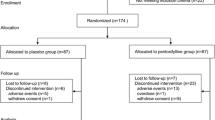
The distribution of patients in the trial is shown in Fig. 1. A total 62 of the 71 initially screened patients met the inclusion criteria and were randomly assigned to PTX add-on losartan and high-dose losartan groups. One patient of the PTX group was missed during the follow-up. Five patients of the high-dose losartan were lost to follow-up; one of them discontinued the treatment and four of them were missed during the trial.
In total, 56 patients completed the 12 weeks of trial. Thirty and 26 patients remained in the trial in PTX add-on losartan and high-dose losartan groups, respectively. The baseline demographics, clinical, and laboratory characteristics of the two groups had no significant differences (Table 1).
Changes in “Pentoxifylline Add-On Losartan” Arm
UAE declined compared with baseline during the trial (mean difference [95% CI] − 161.1 [− 216.91, − 105.3], p < 0.001). hs-CRP was another marker that reduced significantly (mean difference [95% CI] − 1.3 [− 1.87, 0.73], p < 0.001). There was also a significant reduction in HbA1c (mean difference [95% CI] − 0.28 [− 0.45, − 0.11], p = 0.002). There were no significant changes in HSP70, TNFα, copeptin, SBP, and DBP compared to baseline (all p values were more than 0.05) (Table 2).
Changes in “High-Dose Losartan” Arm
Similar to the PTX group, UAE, hs-CRP, and HbA1c decreased significantly in this arm (mean difference [95% CI] − 79.36 [− 105.07, − 53.65] (p < 0.001), − 0.53 [− 0.80, − 0.26] (p < 0.001), and − 0.2 [− 0.34, − 0.05] (p = 0.008), respectively). In contrast to the PTX group, there were significant decreases in copeptin and TNFα serum levels (mean difference [95% CI] − 95 [− 171.7, − 18.3] (p = 0.017) and − 30 [− 59.2, − 0.98] (p = 0.043), respectively). Moreover, we observed a significant reduction in FPG, SBP, and DBP (mean difference [95%] CI − 12.9 [− 24.3, − 1.36] (p = 0.03), − 7.9 [− 10.98, − 4.82] (p < 0.001), and − 3.8 [− 6.1, − 1.57] (p = 0.002), respectively) (Table 2).
Comparative Efficiency of Interventions
The comparative effects of the two treatment arms in reducing target outcome measures were assessed with ANCOVA modelling and the results are presented in Table 3. The PTX intervention was associated with a superior reduction of UAE and hs-CRP levels compared with the losartan intervention (p = 0.018, effect size 9.1%; p = 0.028, effect size 9.2%, respectively) and the effect size increased in multivariable adjusted model 4 (model 4: p = 0.012, effect size 11.3%; p = 0.02, effect size 11.4%, respectively). The losartan arm was more successful in reducing TNFα, copeptin, SBP, and DBP values compared with the PTX arm in the baseline ANCOVA model and after controlling for possible confounders in multivariable adjusted models (baseline model: p = 0.045, effect size 7.3%; p = 0.018, effect size 10.1%; p = 0.001, effect size 23%; p = 0.012, effect size 10.2%, respectively) and the effect size increased in multivariable adjusted model 4 (model 4: p = 0.022, effect size 10.7%; p = 0.021, effect size 10.8%; p = 0.001, effect size 25%; p = 0.007, effect size 12.6%, respectively). The losartan arm was more efficient in reducing HSP70 compared with the PTX arm after controlling for possible confounders in multivariable adjusted models (model 2: p = 0.046, effect size 4.7%, model 3: p = 0.041, effect size 4.7%; and model 4: p = 0.039, effect size 8.6%) (Table 3). There was no significant difference in HbA1c reduction between the two groups (p = 0.96).
Discussion
This study explored the cooperative effects of pentoxifylline add-on losartan and high dose of losartan on serum markers of diabetic nephropathy after 3 months of intervention. Patients in the pentoxifylline experienced comparatively superior reductions in serum hs-CRP levels and UAE rates, and patients in the losartan arm recorded larger reductions in HSP70, TNFα, copeptin, SBP, and DBP measures.
Heat shock proteins (HSP) have protective effects against stressful conditions [29], and there are abnormalities of HSPs in patients with type 2 diabetes [30]. Nakhjavani et al. showed that HSP70 is increased in patients with type 2 diabetes and could be a potential diagnostic factor in such patients [30]. Recent studies showed that HSP molecules play a role in the pathogenesis of diabetic nephropathy [31, 32], and there is an increase in serum HSP70 levels in patients with albuminuria [8]. Plasma copeptin is a surrogate marker of vasopressin associated with a decline in kidney function and progression of diabetic nephropathy in patients with type 1 and type 2 diabetes [33, 34]. Inflammatory parameters are increased in patients with diabetes, and both serum and urinary inflammatory parameters are independently associated with proteinuria in diabetic nephropathy [35]. Indeed, diabetes is now considered as an inflammatory disease. hs-CRP is a sensitive marker of chronic inflammation [36], and studies have shown that there is an independent association between hs-CRP and UAE in patients with diabetes [35, 37, 38]. It has been known for several years that ACEIs and ARBs reduce UAE through blocking the renin–angiotensin–aldosterone system (RAAS) and thus have a slowing effect on the progression of diabetic nephropathy [39]. The use of ACEI or ARB is currently the first step in the strategy to reduce proteinuria in patients with type 1 and type 2 diabetes [20, 40]. The linkage between diabetes and inflammation has generated interest in anti-inflammatory therapies to slow diabetes and the associated nephropathy progression [41]. Pentoxifylline is a non-specific phosphodiesterase inhibitor with known anti-inflammatory and antifibrotic effects [42]. Potential mechanisms of pentoxifylline action on diabetic nephropathy are manifold. Phosphodiesterase (PDE) is the main enzyme in the synthesis of pro-inflammatory cytokines [43]. Therefore, the inhibitory effect of PTX on PDEs results in the reduction of inflammatory markers such as TNFα, hs-CRP, IL-6, and interferon-α [44]. Several studies have shown that PTX has protective effects on renal function and reduces UAE rates in patients with diabetes [45,46,47,48,49], but there are only a few studies that evaluate the effect of PTX on serum markers of diabetic nephropathy and glycemic indices.
Alidadi et al. conducted a randomized clinical trial in patients on hemodialysis who received either PTX or placebo [50]. They found a significant decrease in serum CRP levels compared to the placebo. Similarly, we found a superior reduction in hs-CRP in the PTX arm. Rabizadeh et al. conducted a randomized trial and assessed the effect of PTX on NT-pro BNP, hs-CRP, and UAE in patients with type 2 diabetes and nephropathy. In agreement with our results, they showed that the PTX arm had a larger reduction in hs-CRP and UAE [51].
Contrary to our expectations, we failed to observe a significant reduction in serum TNFα in the PTX group. Similarly, Han et al. also failed to show any significant changes in serum TNFα in patients with diabetic nephropathy in a randomized clinical trial [19]. A meta-analysis of the use of PTX for treating nonalcoholic fatty liver disease also failed to show significant changes in serum TNFα levels compared with placebo [52]. Another study by Chen et al. showed no significant changes in TNFα during the administration of PTX 800 mg daily in patients with proteinuric primary glomerular diseases [53]. Moreover, Akbari et al. showed that use of PTX in inflammatory processes was a double-edged sword and increased the expression of inflammatory genes in the rat hippocampus as a result of its vasodilatory effects [54].
Conclusion
Treatment with PTX add-on losartan in comparison with high-dose losartan monotherapy was more effective in reducing hs-CRP and UAE. In addition, high-dose losartan monotherapy significantly reduced TNFα, SBP, and DBP. In conclusion, add-on PTX to losartan may be a more effective approach to reduce residual albuminuria and inflammation compared to high-dose losartan alone in the management of diabetic nephropathy, although further studies are needed to evaluate the effect of add-on PTX on serum markers of diabetic nephropathy.
References
Lin YC, Chang YH, Yang SY, Wu HD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018;117(8):662–75.
Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2015;5(1):49–56.
MacIsaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63(Supplement 2):S39–62.
Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24(11):3265–8.
Skálová S. The diagnostic role of urinary N-acetyl-beta-d-glucosaminidase (NAG) activity in the detection of renal tubular impairment. Acta Medica (Hradec Kralove). 2005;48(2):75–80.
Kamijo A, Sugaya T, Hikawa A, et al. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165(4):1243–55.
Fiseha T, Tamir Z. Urinary markers of tubular injury in early diabetic nephropathy. Int J Nephrol. 2016;2016:4647685.
Morteza A, Nakhjavani M, Larry M, Nargesi AA, Esteghamati A. Heat shock protein 70 and albuminuria in patients with type 2 diabetes: a matched case control study. Cell Stress Chaperones. 2013;18(6):815–9.
Velho G, Ragot S, El Boustany R, et al. Plasma copeptin, kidney disease, and risk for cardiovascular morbidity and mortality in two cohorts of type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):110.
Sommese L, Zullo A, Mancini FP, Fabbricini R, Soricelli A, Napoli C. Clinical relevance of epigenetics in the onset and management of type 2 diabetes mellitus. Epigenetics. 2017;12(6):401–15.
Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond). 2009;33(1):54–66.
Husain K, Hernandez W, Ansari RA, Ferder L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J Biol Chem. 2015;6(3):209–17.
Susic D, Varagic J. Obesity: a perspective from hypertension. Med Clin North Am. 2017;101(1):139–57.
Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39 Suppl 2:S244–52.
González-Espinoza L, Rojas-Campos E, Medina-Pérez M, Peña-Quintero P, Gómez-Navarro B, Cueto-Manzano AM. Pentoxifylline decreases serum levels of tumor necrosis factor alpha, interleukin 6 and C-reactive protein in hemodialysis patients: results of a randomized double-blind, controlled clinical trial. Nephrol Dial Transplant. 2012;27(5):2023–8.
El-Haggar SM, Eissa MA, Mostafa TM, El-Attar KS, Abdallah MS. The Phosphodiesterase inhibitor pentoxifylline as a novel adjunct to antidepressants in major depressive disorder patients: A proof-of-concept, randomized, double-blind, placebo-controlled trial. Psychother Psychosom. 2018;87(6):331–9.
Brie D, Sahebkar A, Penson PE, et al. Lipid, Blood pressure meta-analysis Collaboration (LBPMC) Group. Effects of pentoxifylline on inflammatory markers and blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2016;34(12):2318–29.
McCarty MF, O’Keefe JH, DiNicolantonio JJ. Pentoxifylline for vascular health: a brief review of the literature. Open Heart. 2016;3(1):e000365–e000365.
Han SJ, Kim HJ, Kim DJ, et al. Effects of pentoxifylline on proteinuria and glucose control in patients with type 2 diabetes: a prospective randomized double-blind multicenter study. Diabetol Metab Syndr. 2015;7:64.
Quiroga B, Arroyo D, de Arriba G. Present and future in the treatment of diabetic kidney disease. J Diabetes Res. 2015;2015:801348–801348.
McCormick BB, Sydor A, Akbari A, Fergusson D, Doucette S, Knoll G. The effect of pentoxifylline on proteinuria in diabetic kidney disease: a meta-analysis. Am J Kidney Dis. 2008;52(3):454–63.
Navarro-González JF, Muros M, Mora-Fernández C, Herrera H, Meneses B, García J. Pentoxifylline for renoprotection in diabetic nephropathy: the PREDIAN study. Rationale and basal results. J Diabetes Complications. 2011;25(5):314–9.
Donate-Correa J, Tagua VG, Ferri C,et al. Pentoxifylline for renal protection in diabetic kidney disease. a model of old drugs for new horizons. J Clin Med. 2019;8(3):287.
Navarro-González JF, Sánchez-Niño MD, Donate-Correa J, et al. Effects of pentoxifylline on soluble klotho concentrations and renal tubular cell expression in diabetic kidney disease. Diabetes Care. 2018;41(8):1817–1820.
Perez-Gomez MV, Sanchez-Niño MD, Sanz AB, et al. Targeting inflammation in diabetic kidney disease: early clinical trials. Expert Opin Investig Drugs. 2016;25(9):1045–58.
Navarro JF, Mora C, Muros M, García J. Additive antiproteinuric effect of pentoxifylline in patients with type 2 diabetes under angiotensin II receptor blockade: a short-term, randomized, controlled trial. J Am Soc Nephrol. 2005;16(7):2119–26.
Tian ML, Shen Y, Sun ZL, Zha Y. Efficacy and safety of combining pentoxifylline with angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker in diabetic nephropathy: a meta-analysis. Int Urol Nephrol. 2015;47(5):815–22.
Zhong B. How to calculate sample size in randomized controlled trial? J Thorac Dis. 2009;1(1):51–4.
Li Z, Srivastava P. Heat-shock proteins. Curr Protoc Immunol 2004. Appendix 1, p. Appendix 1T.
Nakhjavani M, Morteza A, Khajeali L, et al. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010;15(6):959–64.
Calabrese V, Mancuso C, Sapienza M, et al. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12(4):299–306.
Buraczynska M, Swatowski A, Buraczynska K, Dragan M, Ksiazek A. Heat-shock protein gene polymorphisms and the risk of nephropathy in patients with Type 2 diabetes. Clin Sci (Lond). 2009;116(1):81–6.
Roussel R, Matallah N, Bouby N, et al. Plasma copeptin and decline in renal function in a cohort from the community: the prospective D.E.S.I.R. study. Am J Nephrol. 2015;42(2):107–14.
Tasevska I, Enhörning S, Christensson A, Persson M, Nilsson PM, Melander O. Increased Levels of Copeptin, a Surrogate Marker of Arginine Vasopressin, are associated with an increased risk of chronic kidney disease in a general population. Am J Nephrol. 2016;44(1):22–8.
Navarro JF, Mora C, Maca M, Garca J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis. 2003;42(1):53–61.
Festa A, D'Agostino R Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102(1):42–7.
Festa A, D'Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The Insulin Resistance Atherosclerosis Study. Kidney Int. 2000;58(4):1703–10.
Jager A, van Hinsbergh VW, Kostense PJ, et al. C-reactive protein and soluble vascular cell adhesion molecule-1 are associated with elevated urinary albumin excretion but do not explain its link with cardiovascular risk. Arterioscler Thromb Vasc Biol. 2002;22(4):593–8.
Sonkodi S, Mogyorósi A. Treatment of diabetic nephropathy with angiotensin II blockers. Nephrol Dial Transplant. 2003;18(suppl_5):v21–3.
KDOQI Clinical Practice Guideline for Diabetes and CKD. 2012 update. Am J Kidney Dis. 2012;60(5):850–86.
Agrawal NK, Kant S. Targeting inflammation in diabetes: newer therapeutic options. World J Diabetes. 2014;5:697–710.
Kapoor S. The renoprotective effects of pentoxifylline: beyond its role in diabetic nephropathy. Korean J Intern Med. 2013;28(3):374–5.
Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109(3):366–98.
Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987;34(1):50–97.
Oliaei F, Hushmand S, Khafri S, Baradaran M. Efficacy of pentoxifylline for reduction of proteinuria in type II diabetic patients. Caspian J Intern Med. 2011;2(4):309–13.
Ghorbani A, Omidvar B, Beladi-Mousavi SS, Lak E, Vaziri S. The effect of pentoxifylline on reduction of proteinuria among patients with type 2 diabetes under blockade of angiotensin system: a double blind and randomized clinical trial. Nefrologia. 2012;32(6):790–6.
Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol. 2015;26(1):220–9.
Kuo KL, Hung SC, Liu JS, Chang YK, Hsu CC, Tarng DC. Add-on protective effect of pentoxifylline in advanced chronic kidney disease treated with renin-angiotensin-aldosterone system blockade - a nationwide database analysis. Sci Rep. 2015;5:17150.
Rodriguez-Morán M, González-González G, Bermúdez-Barba MV, et al. Effects of pentoxifylline on the urinary protein excretion profile of type 2 diabetic patients with microproteinuria: a double-blind, placebo-controlled randomized trial. Clin Nephrol. 2006;66(1):3–10.
Alidadi A, Fard RG. Effect of pentoxifylline on serum CRP levels in hemodialysis patients compared with placebo. Int J Pharm Sci Res. 2018;9(6):2347–50.
Rabizadeh S, Dehghani Firouzabadi F, Noshad S, et al. Beneficial effects of Pentoxifylline plus losartan dual therapy in type 2 diabetes with nephropathy. Am J Med Sci. 2018;355(5):442–8.
Zeng T, Zhang CL, Zhao XL, Xie KQ. Pentoxifylline for the treatment of nonalcoholic fatty liver disease: a meta-analysis of randomized double-blind, placebo-controlled studies. Eur J Gastroenterol Hepatol. 2014;26(6):646–53.
Chen YM, Lin SL, Chiang WC, Wu KD, Tsai TJ. Pentoxifylline ameliorates proteinuria through suppression of renal monocyte chemoattractant protein-1 in patients with proteinuric primary glomerular diseases. Kidney Int. 2006;69(8):1410–5.
Akbari Z, Reisi P, Torkaman-Boutorabi A, Farahmandfar M. The effect of pentoxifylline on passive avoidance learning and expression of tumor necrosis factor-alpha and caspase-3 in the rat hippocampus following lipopolysaccharide-induced inflammation. Adv Biomed Res. 2019;8:39.
Acknowledgements
Research reported in this publication was supported by Elite Researcher Grant Committee under award number 982876 from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Funding
This publication was supported by Elite Researcher Grant Committee under award number 982876 from the National Institutes for Medical Research Development (NIMAD). No funding was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
A.E. and S.R. contributed to conception. F.M., A.M., and F.D.F contributed to data analysis. A.S. and A.F. contributed to drafting. F.M. and M.N. contributed to revision.
Disclosures
Fatemeh Moosaie, Soghra Rabizadeh, Aida Fallahzadeh, Ali Sheikhy, Alipasha Meysamie, Fatemeh Dehghani Firouzabadia, Manouchehr Nakhjavania, and Alireza Esteghamatiadeclare have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol is available in the Iranian Registry of Clinical Trials (IRCT 20121104011356N10) (https://en.irct.ir/trial/46758) and use of human data was in accordance with guidelines of the Helsinki Declaration of 1964 and its later amendments. Written informed consent was obtained from each subject regarding the privacy and anonymity of data collection. All individuals’ experiments were approved by the National Institute for Medical Research Development (NIMAD) ethical committee (IR.NIMAD.REC.1398.193).
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Moosaie, F., Rabizadeh, S., Fallahzadeh, A. et al. Effects of Pentoxifylline on Serum Markers of Diabetic Nephropathy in Type 2 Diabetes. Diabetes Ther 13, 1023–1036 (2022). https://doi.org/10.1007/s13300-022-01250-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01250-y