Abstract
Introduction
The aim of this study was to evaluate change in laboratory-measured HbA1c in patients with either type 1 diabetes (T1D) or type 2 diabetes (T2D) on insulin therapy following initiation of the FreeStyle Libre™ flash glucose monitoring system.
Methods
This was a retrospective observational study on adults with T1D or T2D on insulin, who were started on the FreeStyle Libre system as part of standard care. HbA1c was recorded at initiation and at 3-month intervals thereafter.
Results
The analysis included 131 patients with T1D and 176 patients with T2D on insulin. Mean HbA1c decreased significantly by 3 months following initiation of the FreeStyle Libre system, both in T1D (− 0.75%, p < 0.001) and in T2D (− 0.54%, p < 0.001). Reductions were maintained for 12 months. Change from 3 to 12 months was not significant either in T1D or T2D. Subgroup analysis showed significant reduction in patients with a baseline HbA1c > 7.5–10%, both in T1D (− 0.59%, p < 0.001) and in T2D on insulin (− 0.62%, p < 0.005) at 12 months. Reductions for subjects with HbA1c > 10% were − 4.66% in T1D and − 3.73% in T2D. No change was seen for subjects with a baseline HbA1c ≤ 7.5% (58 mmol/mol). Linear regression confirms that baseline HbA1c is strongly negatively correlated with subsequent change in HbA1c in T1D and in T2D.
Conclusions
Patients with T1D or T2D show a reduction in HbA1c by 3 months following initiation of the FreeStyle Libre system. The mean fall in HbA1c at 3 months is strongly negatively correlated with starting HbA1c. This reduction is maintained over 12 months. The significant benefit is seen in patients with a starting HbA1c > 7.5% (58 mmol/mol).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
To evaluate change in HbA1c over 12 months in people with either type 1 diabetes or type 2 diabetes on insulin in real-world care in Germany following initiation of the FreeStyle Libre™ flash glucose monitoring system. |
To evaluate the correlation between change in HbA1c over 12 months following initiation of the FreeStyle Libre™ system with HbA1c at baseline. |
To evaluate the relationship between change in HbA1c with prior metabolic control following initiation of the FreeStyle Libre™ system. |
What was learned from the study? |
In real-world diabetes care in Germany, HbA1c is significantly reduced in people with T1D (− 0.75%) or T2D (− 0.54%) on insulin after initiation of the FreeStyle Libre™ system (p < 0.001 in both cases). |
The reduction in HbA1c is achieved within 3 months of initiation and is sustained over 12 months. |
The most significant benefit is seen in patients with poorer metabolic control, with baseline HbA1c levels > 7.5% (58 mmol/mol) prior to initiation of the FreeStyle Libre™ system. |
Baseline HbA1c is negatively correlated with subsequent change in HbA1c with the FreeStyle Libre™ system. In T1D mean HbA1c falls by 0.72% for each 1% increase in baseline HbA1c. In T2D on insulin, mean HbA1c falls by 0.71% for each 1% increase in baseline HbA1c. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13281419.
Introduction
HbA1c is the established gold-standard indicator for assessing long-term glucose control in diabetes. Landmark clinical trials, including the UK Prospective Diabetes Study (UKPDS) [1] and the Diabetes Control and Complications Trial (DCCT) [2], have established that a lower HbA1c is associated with clinically significant reductions in the incidence of microvascular complications and long-term macrovascular disease. Real-time continuous glucose monitoring (rtCGM) devices have been shown to help patients with type 1 diabetes (T1D) or type 2 diabetes (T2D) on insulin therapy to reduce HbA1c levels [3]. As well as reducing laboratory-measured HbA1c levels, randomised clinical trials (RCTs) indicate that there is also a reduction in episodes of hypoglycaemia and glycaemic variability with rtCGM devices, in comparison to self-monitored blood glucose (SMBG) testing [4,5,6,7,8].
The FreeStyle Libre™ system (Abbott Diabetes Care, Witney, UK) is a factory-calibrated interstitial glucose monitoring system in which a sensor is applied to the upper arm and collects glucose readings over a 14-day wear period [9]. The sensor automatically measures glucose every minute and readings are stored in 15-min intervals. Glucose readings are not automatically communicated to the FreeStyle Libre reader, rather the data is collected when the user scans the sensor either with the reader or a smartphone app [10]. This type of CGM is also known as intermittently scanned CGM (isCGM) or flash glucose monitoring. Two RCTs of the FreeStyle Libre system in T1D and T2D on insulin have shown significant reductions in time in hypoglycaemia without demonstrating a significant change in HbA1c levels [11, 12]. In contrast, recent prospective studies have shown improvements in HbA1c for children and adults using the FreeStyle Libre system in T1D and for adults with T2D on insulin [13,14,15,16,17,−18].
Here we report on the experience of two treatment centres in Germany, where the FreeStyle Libre system was introduced to patients with either T1D or T2D on insulin as part of standard care, and HbA1c values recorded over 12 months following initiation.
Methods
Study Selection Criteria and Outcomes Measure
This retrospective observational analysis was performed on data collected at two German clinical centres, the Gemeinschaftspraxis Drs. Klausmann in Aschaffenburg and Zentrum für Diabetes und Gefäßerkrankungen Münster. Both centres are established in delivering standard outpatient care for people with diabetes within the German healthcare system. De-identified patient records were examined to select subjects with either T1D or T2D on insulin who were initiated on the FreeStyle Libre system as part of standard care. No selection criteria were applied other than treatment with FreeStyle Libre as part of standard care. The data reflect consecutive adult patients started on FreeStyle Libre between November 2015 and September 2018. No FreeStyle Libre sensor-derived glucose data is reported in this study. Laboratory tested HbA1c values were recorded for all patients prior to the start of FreeStyle Libre using standard clinical laboratory reference analyzers, with at least one HbA1c value that was established after starting. Not all subjects had data recorded at each interval across the 12-month analysis period, as a consequence of the time of their start of FreeStyle Libre or a missed attendance.
Statistics
Matched paired data were analysed using both the data analysis tools in Microsoft Excel 2016 and the R Project for Statistical Computing (www.r-project.org) software version 3.6.2. The level of significance was set at 0.05 or better. A linear model was used to investigate the trend of mean HbA1c values across the measurement time points from baseline onwards. Student’s t test was used to compare means of matched paired data and unmatched data as appropriate to the analysis. Tukey’s contrast analysis was used to compare the means of every outcome time point from 3 months onwards. Linear regression was used to identify the predicted change in HbA1c given the input baseline HbA1c.
Compliance with Ethics Guidelines
This retrospective observational study used only existing de-identified electronic medical record data that were collected in daily clinical practice. No intervention was implemented on the subjects for the purpose of the study, and no patient-identifiable information was used in the study. The study was compliant with the Helsinki Declaration of 1964, and its later amendments.
Results
Inclusion Criteria and Baseline Characteristics
A total of 131 patients with T1D and 176 patients with T2D on insulin met the inclusion criteria and were included in the analysis. The age of patient ranged from 24 to 92 years. All patients were recorded as being on insulin therapy for the duration of the analysis, either on multiple daily doses of insulin (MDI), mealtime insulin only or continuous subcutaneous insulin infusion (CSII). The baseline characteristics of the study population are provided in Table 1.
Change in Laboratory Measured HbA1c
A statistically significant reduction in mean HbA1c from baseline was detected at all time points in 131 patients with T1D (Fig. 1). Mean starting baseline (± SE) was 8.15% (± 0.15%). HbA1c values decreased by − 0.75% (± 0.15%) at 3 months, by − 0.76% (± 0.17%) at 6 months, by − 0.72% (± 0.19%) at 9 months and by 0.74% (± 0.21%) at 12 months (p < 0.001 in all cases; Fig. 1). A similar trend was seen in 176 patients with T2D on insulin (Fig. 2), with a mean baseline HbA1c of 7.76% (± 0.12%). HbA1c was reduced by − 0.54% (± 0.11%) at 3 months, by 0.43% (± 0.11%) at 6 months, by − 0.39% (± 0.13%) at 9 months and by − 0.38% (± 0.17%) at 12 months (P < 0.001 at 3 and 6 months, P < 0.002 at 9 months; P = 0.014 at 12 months; Fig. 2).
Mean HbA1c levels for 131 patients with type 1 diabetes at 3-monthly intervals, following initiation of the FreeStyle Libre flash glucose monitoring system. Each time point contains the data for the cohort of study subjects with both a baseline and an intervention HbA1c reading for that time point. All subjects (n = 131) had a baseline HbA1c, but not all subjects provided HbA1c data for all time points thereafter. The data shown in the table is the mean baseline and HbA1c at each time point, and the mean change in HbA1c. Data are shown as mean HbA1c (%) ± standard error (SE). Patients experienced a statistically significant reduction in HbA1c levels from baseline, which was sustained over 12 months. Differences in mean HbA1c were not significant between 3 and 12 months
Mean HbA1c levels for 176 patients with type 2 diabetes at 3-monthly intervals, following initiation of the FreeStyle Libre flash glucose monitoring system. Each time point contains the data for the cohort of study subjects with both a baseline and an intervention HbA1c reading for that time point. All subjects (n = 176) had a baseline HbA1c, but not all subjects provided HbA1c data for all time points thereafter. The data shown in the table is the mean baseline and HbA1c at each time point, and the mean change in HbA1c. Data are shown as mean HbA1c (%) ± standard error (SE). Patients experienced a statistically significant reduction in HbA1c levels from baseline, which was sustained over 12 months. Differences in mean HbA1c were not significant between 3 and 12 months
Tukey contrast analysis both in T1D and T2D showed that the difference between time points after 3 months was not significant, indicating that the greatest impact on HbA1c values was observed within the first 3 months of use of the FreeStyle Libre system and sustained for 12 months.
Change in HbA1c in Patients Stratified by Baseline Metabolic Control
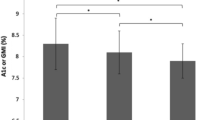
In a subgroup analysis centred on metabolic control, we stratified patients into those with baseline HbA1c ≤ 7.5% (58 mmol/mol), those with baseline HbA1c > 7.5–10% (> 58–86 mmol/mol) and those with HbA1c > 10% (> 86 mmol/mol). This showed that patients with T1D or T2D on insulin and with a baseline HbA1c > 7.5% (> 58 mmol/mol) achieved a significant reduction in HbA1c over time with FreeStyle Libre, whereas those with HbA1c levels ≤ 7.5% (58 mmol/mol; Fig. 3a, b) did not. For all patients with HbA1c in the range > 7.5–10% (> 58–86 mmol/mol) the change at 12 months was significant but was considerably greater amongst patients with HbA1c > 10% (86 mmol/mol). For people with T1D, those with mean HbA1c > 7.5–10% (> 58–86 mmol/mol) achieved a clinically significant reduction of 0.59% (± 0.19%) after 12 months, from a mean HbA1c from 8.49% to 7.90% (Fig. 3a; p < 0.01). For those with a baseline HbA1c > 10% (86 mmol/mol) there was a mean 4.66% (± 0.87%) reduction, from 11.83% to 7.17% (Fig. 3a; p < 0.001). In people with T2D on insulin and HbA1c > 7.5–10% (> 58–86 mmol/mol), the reduction in mean HbA1c 12 months after starting FreeStyle Libre was 0.62% (± 0.22%), from 8.43 to 7.81% (Fig. 3b; p < 0.01) and for patients with HbA1c > 10% (86 mmol/mol) the reduction at 12 months was 3.73% (from 11.4% to 7.67%; p < 0.01).
Mean change in HbA1c over 12 months for patients with baseline HbA1c ≤ 7.5%, HbA1c > 7.5–10% and HbA1c > 10%. *p < 0.001 for difference in mean change in HbA1c. †p < 0.01 for difference in mean change in HbA1c from baseline. §p = 0.012 for difference in mean change in HbA1c from baseline. Data are shown as mean change in HbA1c (%) ± standard error (SE). A significant decrease in HbA1c is observed in patients with a baseline HbA1c > 7.5% (58 mmol/mol) for all time points (p < 0.001), whereas those with a baseline HbA1c ≤ 7.5% (58 mmol/mol) did not demonstrate a significant decrease
Relationship Between Baseline HbA1c and Change in HbA1c at 3 months After Starting FreeStyle Libre
Linear regression was used to identify the predicted change in HbA1c at 3 months, given the input baseline HbA1c. This confirms that baseline HbA1c is strongly negatively correlated with subsequent change in HbA1c, both in T1D (R2 = 0.602, p < 0.001; Fig. 4a) and in T2D (R2 = 0.698, p < 0.001; Fig. 4b). In T1D, on average, for each percentage increase in mean initial HbA1c, the mean change in final HbA1c at 3 months falls by an additional 0.72% (95% CI − 0.83 to − 0.62). In T2D, for each percentage increase in mean initial HbA1c, the mean change in final HbA1c falls by an additional 0.71% (95% CI − 0.79 to − 0.64). No correlation was seen between change in HbA1c and age at diagnosis, age at intervention or duration of diabetes (data not shown).
Discussion
Our retrospective observational analysis of diabetes management in a real-world setting shows that there is a significant and rapid reduction in HbA1c in patients with either T1D or T2D on insulin following the introduction of the FreeStyle Libre system to their standard care. These outcomes differ from two RCTs previously undertaken to evaluate the impact of the FreeStyle Libre system. In both the IMPACT [11] study in patients with T1D and the REPLACE [12] study in patients with T2D on insulin, no significant change was demonstrated in HbA1c levels over 26 weeks when the FreeStyle Libre system was compared with SMBG. In IMPACT, the study population was well controlled, with a baseline mean HbA1c < 7.5% (58 mmol/mol), whereas in REPLACE the baseline mean HbA1c was > 8.5% (64 mmol/mol). Only in a pre-specified subgroup analysis of patients under 65 years in the REPLACE study was a significant change in HbA1c seen compared with SMBG controls (− 0.33%; p = 0.0301) [12]. In contrast, the single-arm SELFY study [13] in a paediatric population with T1D demonstrated a significant reduction in HbA1c (− 0.4%, p < 0.001) when using the FreeStyle Libre system compared to SMBG over 8 weeks. Recent prospective data from 107 adult subjects with T1D in Belgium [16] demonstrated a reduction in HbA1c of − 0.74% after 3 months following introduction of the FreeStyle Libre system. A larger prospective study on adults with T1D in Scotland [15] has demonstrated that 48.1% of 750 subjects achieved a reduction in HbA1c of 0.5% or more over a median 245 days (8 months) following introduction of the FreeStyle Libre system. In T2D, a prospective study in two centres in Israel on 101 adults with T2D on insulin demonstrated a − 0.49% reduction in HbA1c from baseline over 10 weeks compared to subjects assigned to the intervention arm using the FreeStyle Libre system (p = 0.005) [14] and a recent retrospective chart-review study across 18 European diabetes centres showed a reduction of − 0.9% in HbA1c for people with T2D managed by basal-bolus insulin 3–6 months after starting the FreeStyle Libre system [18].
The outcomes from two German diabetes treatment centres reported here confirm these improvements in HbA1c for patients with either T1D or T2D on insulin. The reductions in HbA1c occur within the first 3 months and are sustained over a 12-month period. Linear regression shows that the major predictor of a reduction in HbA1c after starting the FreeStyle Libre system is HbA1c at baseline. For each percentage increase in mean initial HbA1c, the mean change in final HbA1c at 3 months in T1D falls by an additional 0.72%, and by 0.71% in T2D on insulin. The subgroup analysis of subjects based on prior metabolic control (Fig. 3) showed that a clinically significant reduction in HbA1c from baseline is achievable for people with T1D or T2D on insulin with mean HbA1c > 7.5–10% (> 58–86 mmol/mol) after starting the FreeStyle Libre system, with greater reductions for patients with HbA1c above 10% (> 86 mmol/mol). People with T1D or T2D on insulin and good prior glucose control (mean HbA1c ≤ 7.5% at baseline) do not see a significant change in their HbA1c over 3–12 months.
Despite the lack of change in HbA1c, those patients with tighter long-term glucose control, as evidenced by a starting HbA1c level below 7.5%, are likely to be improving their metabolic control using the FreeStyle Libre system, but not by reducing the HbA1c. The outcomes from the IMPACT trial [11] for patients with T1D and in the REPLACE trial [12] for subjects with T2D on MDI indicate that all patients using FreeStyle Libre can expect to significantly reduce both their time in hypoglycaemia below 70 mg/dL (3.9 mmol/L) and below 54 mg/dL (3.0 mmol/L), as well as reducing their frequency of low-glucose events. This level of analysis in the current study would require inclusion of sensor-dependent glucose data which is not possible in the setting of this retrospective observational study.
The observation that change in HbA1c happens within the first 3 months is not surprising. Many studies have shown that reductions in a range of glycemic measures, including HbA1c, rapidly follow the introduction both of rtCGM or the FreeStyle Libre system as part of diabetes care. A recent meta-analysis of 29 RCTs or real-world studies in children and adults with T1D or T2D showed that the significant reductions in HbA1c following initiation of the FreeStyle Libre system were seen between 2 and 4 months after starting [17]. This is postulated to be a consequence of the immediate access for users of the FreeStyle Libre system to a range of glycemic information that can improve their decision-making during daily diabetes self-care. These include their glucose status in real time, the trend arrows that indicate the direction and speed of change in their glucose status [19] and the summary reports that are available to them via the readers or smartphone apps that they use to scan and collect glucose data. This information facilitates an in-depth awareness of their daily life and allows for effective treatment decisions that are not possible with SMBG testing. The intuitive nature of CGM systems mean that this improvement in self-care behaviour starts following the application of the first glucose sensor and is sufficient for a change in long-term HbA1c to be evident after 3 months.
It is important to acknowledge that our real-world study has limitations. It is a retrospective chart-review study in which limited additional demographic information is available or presented other than age, diabetes status and duration, and insulin regime. In this context, it must be acknowledged that initiating use of the FreeStyle Libre system is only one of a number of factors that may have had an impact on the observed outcomes. For example, training on the FreeStyle Libre device along with specific diabetes education from their healthcare professionals during the initiation process can result in improved diabetes self-care behaviours independent of the device itself that are not controlled for in this retrospective observational analysis. However, our interpretation is that the benefits of immediate and longer-term awareness of glucose trends and patterns, provided by the FreeStyle Libre system, are empowering many of the users to make better-informed and proactive decisions about daily glucose control, both in terms of their diet and lifestyle, as well as improved management of their insulin therapy. This particularly applies to the subset of patients with poorer glucose control at the point at which the FreeStyle Libre system has been initiated. The strengths of our study are the unrestricted inclusion criteria, the significant number of people with type 2 diabetes on insulin and the use of a single, consistent outcome.
Ultimately, the data reported here adds to the growing real-world evidence highlighting the impact of flash glucose monitoring on glycaemic control for people with T1D or T2D. Importantly, it confirms that people with T2D on insulin therapy see a considerable benefit in long-term HbA1c when managed with flash glucose monitoring. This is in agreement with the outcomes of a much shorter 10-week prospective interventional study on 101 patients with T2D on insulin [14] and also concur with the outcomes of a real-world observational study over 3–6 months, which included 183 adults with T2D managed with basal-bolus insulin from German diabetes centres [18]. Our retrospective observational analysis has demonstrated similar results within a population of patients with T2D on insulin in Germany, over an extended 12-month period. The data presented here is also aligned with previous real-world studies in T1D showing that the greatest reduction in HbA1c is attained by subjects with a higher baseline [14,15,16,17].
Conclusions
The results presented here build on the evidence for improved glucose control, as measured by HbA1c, using flash glucose monitoring in patients with either T1D or T2D on insulin. This retrospective observational analysis shows that the introduction of the FreeStyle Libre system is associated with a significant reduction in HbA1c levels in people with diabetes on insulin within 3 months of initiation and the results are sustainable over 12 months. Furthermore, the most significant benefit regarding HbA1c reduction is seen in patients whose baseline HbA1c levels are above 7.5% (58 mmol/mol). These improvements in glucose control may contribute to a reduction in the long-term risk of microvascular and macrovascular complications and the consequent costs of morbidity and mortality associated with diabetes.
References
Holman RR, Paul SK, Bethel AM, Matthews DR, Neil AH. 10-year follow-up of intensive glucose control in type 2 diabetes. New Engl J Med. 2008;359(15):1577–89. https://doi.org/10.1056/nejmoa0806470.
Nathan D, Genuth S, Lachin J. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med. 1993;329(14):977–86. https://doi.org/10.1056/nejm199309303291401.
Ajjan RA. How can we realize the clinical benefits of continuous glucose monitoring? Diabetes Technol Ther. 2017;19(S2):1–10. https://doi.org/10.1089/dia.2017.0021.
Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. New Engl J Med. 2008;359(14): 1464–1476. https://doi.org/10.1056/nejmoa0805017.
Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017a;317(4):371–8. https://doi.org/10.1001/jama.2016.19975.
Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections. Ann Intern Med. 2017b;167(6):365. https://doi.org/10.7326/m16-2855.
Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs. conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379–87. https://doi.org/10.1001/jama.2016.19976.
Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155–62. https://doi.org/10.1007/s00125-012-2708-9.
Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17(11):787–94. https://doi.org/10.1089/dia.2014.0378.
Blum A. Freestyle libre glucose monitoring system. Clin Diabetes. 2018;36(2):cd170130. https://doi.org/10.2337/cd17-0130.
Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254–63. https://doi.org/10.1016/s0140-6736(16)31535-5.
Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter. Open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55–73. https://doi.org/10.1007/s13300-016-0223-6.
Campbell FM, Murphy NP, Stewart C, Biester T, Kordonouri O. Outcomes of using flash glucose monitoring technology by children and young people with type 1 diabetes in a single arm study. Pediatr Diabetes. 2018;19(7):1294–301. https://doi.org/10.1111/pedi.12735.
Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019:dc180166. https://doi.org/10.2337/dc18-0166.
Tyndall V, Stimson RH, Zammitt NN, et al. Marked improvement in HbA1c following commencement of flash glucose monitoring in people with type 1 diabetes. Diabetologia. 2019;62(8):1349–56. https://doi.org/10.1007/s00125-019-4894-1.
Paris I, Henry C, Pirard F, Gérard A, Colin IM. The new FreeStyle libre flash glucose monitoring system improves the glycaemic control in a cohort of people with type 1 diabetes followed in real-life conditions over a period of one year. Endocrinol Diabetes Metab. 2018;1(3):e00023. https://doi.org/10.1002/edm2.23.
Evans M, Welsh Z, Ells S, Seibold A. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta-analysis of clinical trials and real-world observational studies. Diabetes Ther. 2020;11:83–95. https://doi.org/10.1007/s13300-019-00720-0.
Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11(1):279–91. https://doi.org/10.1007/s13300-019-00741-9.
Ziegler R, von Sengbusch S, Kröger J, et al. Therapy adjustments based on trend arrows using continuous glucose monitoring systems. J Diabetes Sci Technol. 2019;13(4):763–73. https://doi.org/10.1177/1932296818822539.
Acknowledgements
Funding
Funding for this analysis and the journal’s Rapid Service Fee were provided by Abbott Diabetes Care.
Medical Writing, Editorial and Other Assistance
Medical writing and editorial assistance in the preparation of this article was provided by Dr Robert Brines of Bite Medical Consulting. Support for this assistance was funded by Abbott Diabetes Care.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Ludger Rose has participated in advisory panels for Novo Nordisk and acted as a consultant for and is a member of the Association of Statutory Health Insurance Physicians. Gerhard Klausmann has participated in advisory panels for Abbott Diabetes Care. Alexander Seibold is an employee of Abbott Diabetes Care.
Compliance with Ethics Guidelines
This retrospective observational study used only existing anonymized electronic medical record data that were collected in daily clinical practice. No intervention was implemented on the subjects for the purpose of the study, and no patient-identifiable information was used in the study. The study was compliant with the Helsinki Declaration of 1964, and its later amendments.
Data Availability
All data analyzed during this study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rose, L., Klausmann, G. & Seibold, A. Improving HbA1c Control in Type 1 or Type 2 Diabetes Using Flash Glucose Monitoring: A Retrospective Observational Analysis in Two German Centres. Diabetes Ther 12, 363–372 (2021). https://doi.org/10.1007/s13300-020-00978-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00978-9