Abstract
Introduction
Epidemiological studies suggest that statins may promote the development or exacerbation of diabetes, but whether this occurs through inhibition of insulin secretion is unclear. This lack of understanding is partly due to the cellular models used to explore this phenomenon (cell lines or pooled islets), which are non-physiologic and have limited clinical transferability.
Methods
Here, we study the effect of simvastatin on insulin secretion using single-islet cultures, an optimal compromise between biological observability and physiologic fidelity. We develop and validate a microfluidic device to study single-islet function ex vivo, which allows for switching between media of different compositions with a resolution of seconds. In parallel, fluorescence imaging provides real-time analysis of the membrane voltage potential, cytosolic Ca2+ dynamics, and insulin release during perfusion under 3 or 11 mM glucose.
Results
We found that simvastatin reversibly inhibits insulin secretion, even in high-glucose. This phenomenon is very rapid (<60 s), occurs without affecting Ca2+ concentrations, and is likely unrelated to cholesterol biosynthesis and protein isoprenylation, which occur on a time span of hours.
Conclusions
Our data provide the first real-time live demonstration that a statin inhibits insulin secretion in intact islets and that single islets respond differently from cell lines on a short time scale.
Funding
University of Padova, EASD Foundation.
Similar content being viewed by others
Introduction
Statins, hydroxy-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitors, are widely used to lower cholesterol and prevent cardiovascular disease [1, 2]. As diabetes is characterized by high cardiovascular risk, international guidelines recommend that most diabetic patients should receive statin therapy [3–5]. Statins effectively reduce cardiovascular morbidity and mortality in patients with diabetes [6], but epidemiological studies suggest they increase the risk of developing diabetes in healthy individuals [7–10] and may worsen glycemia in diabetic patients [11]. Pathophysiologic studies in humans indicate that statins may affect both insulin sensitivity and insulin secretion [12]. In vitro studies show that statins impair insulin secretion by affecting multiple pathways [13], including cellular cholesterol synthesis, membrane fluidity, and isoprenylation of proteins [14]. Though their cardiovascular benefit exceeds the diabetes hazard [15], the mechanisms whereby statins accelerate the onset of diabetes remain unclear, and whether statins truly exert any action on insulin secretion is debated [16, 17]. This is in part because cellular models used to explore this phenomenon, either cell lines [13, 14] or pooled islets [14], are non-physiologic and lack clinical transferability. Cell lines may behave differently from mature beta cells or have incomplete glucose sensor or insulin secretion machinery, whereas pooled islet cultures are poorly suitable to provide information on dynamic insulin secretion because islet response may be heterogeneous and asynchronous.
A single islet is the minimum fully functional unit of the endocrine pancreas. A precise understanding of islet response to exogenous stimuli is better captured in vitro at the single-islet level, devoid of complex and not fully understood islet–islet interactions. Microfluidics is a technology suitable for cell culture at micrometer scale. Because a single islet has an approximate size of 50–500 µm, it is particularly suitable for microfluidic culture. Accordingly, a number of microfluidic devices have been developed for understanding single islet behavior, as recently reviewed [18]. However the advantages of microfluidic technology go well beyond the size of these devices [19]. For example, Benninger et al. used a microfluidic device to generate precise glucose concentration gradients in a single islet microenvironment to study cell–cell gap junctions in the regulation of coordinated insulin release [20]. Microfluidic devices favor the accumulation of endogenously secreted factors and were previously used to detect islet amyloid polypeptide secretion from as few as ten islets [21] and glucose flux in live myoblasts [22], besides insulin [23].
Here, we study the effects of simvastatin on insulin secretion from single murine islets. A microfluidic system able to temporally control the single-islet soluble microenvironment is presented. The setup has been technically and biologically validated, before analyzing simvastatin action on intact isolated islets.
Methods
Microfluidic Setup
The setup was produced by standard photo- and soft-lithographic techniques. Details on the fabrication of the islet culture and the multi-inlet chips are given in the Electronic Supplementary Material (ESM).
Animals and Pancreatic Islet Extraction and Preparation
The protocol followed the Guide for the Care and Use of Laboratory Animals, eighth edition (2011) (http://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf), and was compliant with local and national laws. For murine islet isolation, we optimized a previously described protocol [24]. Details are given in the ESM.
Microfluidic System Running Conditions
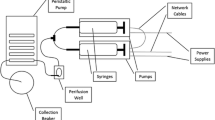
The multi-inlet microfluidic system was connected to medium vials of different compositions. Details are given in ESM. This system allowed switching between up to nine different medium compositions by remotely controlling valve opening and closing. The complete system is displayed in Fig. 1.
Microfluidic setup design and validation. a Full setup for single islet microscopy observation under controlled dynamic biochemical stimulation. A multi-inlet microfluidic chip contains a system of pneumatic valves that are used to temporally control the medium composition entering the islet culture chamber. The pneumatic valve system is actuated by means of electrovalves (not shown) and is responsible for each valve opening and closing according to the mechanism shown in c: pressurizing the control channel prevents medium flow through the flow channel. A single islet is placed in each channel of the islet culture microfluidic chip, where it is precisely positioned because of the presence of a mechanical trap that stops its flow along the channel, as shown in c. The islet culture microfluidic chip is a transparent device that is put under the microscope for online observation. Once the specific valve is open, medium flows through the channel because it is contained in a pressurized vial; to accurately control its flow rate, a syringe pump working in aspiration is placed downstream of the culture system. d The system described in a–c makes possible the biochemical stimulation of a single-islet with temporal accuracy of seconds. Medium composition is changed over time with minimal external perturbation. e Validation of the dynamic input system using medium with and without fluorescein. The theoretical curve (red) is compared with the actual medium fluorescence (green) detected in the region of interest (ROI) indicated. A delay of approximately 50 s was obtained because of the distance between the valve system and the ROI
Islet Staining for Imaging of Viability, Ca2+, Membrane Potential, and Zn2+
Islets were loaded with 1 mg/ml Hoechst 33342 (Sigma-Aldrich) for 10 min. PE-conjugated Annexin-V (BD Biosciences) was added in the perfusion medium. Prior to microfluidic device insertion, islets were loaded with 3-μM Fura Red acetoxymethyl (AM) (ThermoFisher) or 10 μM Fluo-4 AM (ThermoFisher) for 50 min in RPMI medium at 37 °C for intracellular Ca2+ detection; 2-μM FluoZin-3 (ThermoFisher) was added at the same concentration to the different media used for islet stimulation. Extracellular Zn2+ concentration detection was used as an indirect measure for insulin secretion detection, as previously described [21]. To avoid biases due to slight differences in the FluoZin-3 concentration or media autofluorescence, before islet insertion into the microfluidic device, a background profile using different media was established at the beginning of each acquisition and then subtracted to the islet response profile. For voltage imaging, islets were incubated for 20 min at 37 °C in RPMI with Vf2.1.Cl (200 nM, kindly provided by Roger Y. Tsien from University of California at San Diego) [25] and Pluronic F–127 (0.1% w/v) (Sigma-Aldrich).
Confocal Imaging
Fluorophores were excited by light from a 460-nm LED (M470L2, Thorlabs) passing through a T510LPXR filter (Olympus) and directed onto the sample through a full mirror (Chroma) while 512 fluorescence emission was selected by a BA495-540HQ filter (Chroma). All fluorescence images were acquired with a 20× water immersion objective (NA 0.96, Olympus) and projected on a Complementary Metal-Oxide Semiconductor (CMOS) cooled camera (PCO), controlled by in-house developed software. Image sequences of fluorescence were acquired continuously at three frames per second with 100 ms exposure time.
Two-Photon Imaging
Two-photon acquisitions were performed with a modular multiphoton microscope (Bergamo II, Thorlabs) equipped with an 8-kHz resonant scanner, extended-field-of-view collection optics, four independent detection channels in the backward direction, and laser-scanned Dodt contrast in the forward direction. The microscope was coupled to two synchronized pulsed laser beams generated by a Ti:sapphire pump laser (Chameleon Ultra 2, Coherent) and an optical parametric oscillator (Camelion Compact OPO). Two-photon microscopy at 800 nm excitation was used to visualize the intracellular calcium (Fluo-4, green), nuclei (Hoechst 33342, blue), and islet structure simultaneously (transmitted light, gray).
Data Analysis
The analysis software, developed in-house in Matlab environment (Release 14, The MathWorks, Inc., Natick, MA, USA), was designed to plot averaged time responses from specific regions of interest (ROIs) as well as to create false-color animations reporting time-dependent fluorescence intensity variations. False-color images were generated using the hue-saturation-value (HSV) visualization algorithm: hue was used to represent fluorescence changes; saturation was set to 1.0; the value (brightness) carried pixel intensity from a reference image that was either updated on a frame-by-frame basis or obtained as an average over a specified number of frames. The resulting frames so constructed were converted to ordinary RGB images and exported in the Audio Video Interlaced (AVI) proprietary video format of Microsoft Video for Windows applications [26]. FluoZin-3 and Fura Red signals were analyzed independently by playing back images in single-channel mode. ROIs used for Fluo-4 and Fura Red signal analysis were drawn covering the islets, while ROIs for FluoZin-3 signal analysis were drawn outside the islet areas. When Fura Red is excited at 488 nm, its fluorescence emission decreases as Ca2+ concentration increases [27]. For this reason, changes in intracellular Ca2+ were expressed as (F0/F)-1 ratios [27]. Fluo-4 and FluoZin-3 signals were measured as relative changes of fluorescence emission intensity: ΔF/F 0, where F 0 is the pre-stimulus basal fluorescence; ΔF = F – F 0, F is fluorescence at time t.
Compliance with Ethics Guidelines
The institutional review board of the Venetian Institute of Molecular Medicine approved the study. The protocol followed the Guide for the Care and Use of Laboratory Animals, eighth edition (2011) (http://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf). All institutional and national guidelines for the care and use of laboratory animals were followed.
Results
Microfluidic Single-Islet Culture Setup
The precise dynamic perturbation of a single-islet environment with the online measurement of its response is prevented in conventional culture systems, but can be robustly achieved using microfluidic devices. To this aim, we designed a microfluidic system where a single islet can be trapped without mechanical damage in a defined position, dynamically stimulated with media of multiple compositions, and contemporarily observed under a microscope (Fig. 1a–c). Each islet was cultured under constant flow perfusion, a condition that better mimics the highly vascularized environment where islets reside in vivo.
The microfluidic device includes an integrated pneumatic valve system (Fig. 1b) that is remotely controlled and enables switching the medium entering the culture chamber in few seconds, while minimizing other culture perturbations (Fig. 1d). The time required for the new medium to reach the islet position within the channel was estimated using buffer with or without fluorescein. At the 2 μl/min flow rate used in all the experiments, there was an approximate 50 s lag between the medium valve switch and medium change at the islet position (Fig. 1e).
The size and transparency of the microfluidic device make possible the online microscopic observation of the islet response. Overall, the described setup provides maximal information in terms of the spatial and temporal resolution of the collected data.
Biological Validation of the Microfluidic Device
The canonical glucose-stimulated insulin secretion (GSIS) pathway in the β-cell fraction of pancreatic islets involves some well-established sequential steps: glucose is transported into β-cells through plasma membrane glucose transporters and is metabolized, thereby elevating the intracellular ATP/ADP ratio; this event causes the closure of cell-surface ATP-sensitive K+ channels, leading to cell membrane depolarization; plasma membrane voltage-dependent Ca2+ channels are consequently opened, increasing cytosolic Ca2+ concentration and triggering the exocytosis of insulin [28].
To interrogate islet behavior via microscopy, we used multiple fluorescent dyes probing cell viability and specific steps of GSIS pathway. We added to the flowing culture media PE-labeled Annexin V, a fluorophore for detecting early apoptosis. We also loaded the islets, prior to their insertion into the device, with dyes enabling the detection of the intracellular Ca2+ concentration (Fluo-4 or Fura Red) and cell membrane potential (Vf2.1Cl). We used both confocal and two-photon microscopy.
After each islet insertion into the device, we always observed its morphology to verify the integrity of the external membrane (Fig. 2a, b; Online Video 1). Islets that did not show a visibly intact morphology, for example, due to some variability in the extraction process, were discarded. This occurred in less than 5% of the cases and was usually associated with the presence of some dead cells, assessed by annexin V, not functionally responsive, for instance in terms of intracellular Ca2+ oscillations under high-glucose stimulation (Fig. 2c, d).
Two-photon imaging of islet morphology and viability in microfluidics. Islets were pre-loaded with Hoechst 33342 and Fluo-4 for nuclear identification and intracellular Ca2+ detection, respectively. Viability was detected by adding annexin V in the flowing medium. a, b Images of single islets, intact (a) or subjected to mechanical stress (b), within a microfluidic channel (see video 1). From top left: morphology (transmitted light), nuclei (blue), intracellular Ca2+ (green), and mortality (red). c, d Plots of intracellular Ca2+ oscillations in the same islets shown in a, b, respectively, constantly perfused with buffer containing stimulatory glucose concentration (11 mM). Zero-centered raw data. Annexin-V+ cells from the stressed islets do not display oscillatory Ca2+ activity, as detected by Fluo-4
To verify that the microfluidic system did not impair islet functional response, we perfused the islets with high-glucose medium (11 mM), which is stimulatory for insulin secretion. We used islets from mutant mice that express GFP under the insulin promoter in order to identify the β-cells within the islet. In Fig. 3a, we show persistent oscillations of the intracellular Ca2+ at 11 mM. As expected, the oscillations were restricted to β-cells and were not an artifact of the culture system or method of analysis. Moreover, β-cells within the same islet showed a properly synchronized response (Fig. 3b).
Validation of islet functional response at 11 mM glucose. a Green fluorescence intensity of an islet isolated from a Mouse Insulin Promoter (MIP)-GFP mouse, pre-loaded with Fura Red for intracellular Ca2+ detection. b Plots of synchronous intracellular Ca2+ oscillations of cells in the regions of interest (ROIs) indicated in a. ROI 7 refers to non β-cells (GFP-), which do not show Ca2+ oscillatory activity in response to high glucose concentration. c Membrane potential of an islet from a wild-type mouse loaded with the voltage dye Vf2.1.Cl and imaged at the indicated time points during constant perfusion at high glucose. The false-colored image sequence shows fluorescence intensity. d Plot of signal intensity inside ROIs indicated in c. Oscillation phase shift describes the spatial propagation of electrical activity from cell to cell within the islet (see also Video 2). e Simultaneous recording of intracellular Ca2+ and membrane potential in an islet from a wild-type mouse, pre-loaded with Fura Red and Vf2.1.Cl. The false-colored image sequence shows fluorescence intensity. f Plot derived from data shown in e. Intracellular Ca2+ and membrane potential waves propagate with the same periodicity. Ca2+ propagation occurs with slightly delayed phase (see also Video 3)
To verify islet structural integrity also in terms of cell–cell electrical coupling, we then loaded the islets with Vf2.1Cl, a voltage-sensitive fluorescent dye, and performed imaging at high speed. Under high-glucose conditions, we were able to observe the spatial propagation of membrane potential waves throughout the islet (Fig. 3c; Online Video 2), as previously reported [29].
As a last verification of functionality, we loaded the islets with probes for detection of both membrane potential and intracellular Ca2+. Imaging at high speed, we observed the two waves propagating at the same frequency, but with a small delay of intracellular Ca2+ compared to membrane voltage (Fig. 3e, f; Video 3). This result is consistent with Ca2+ regulation occurring along the pathway of insulin secretion and confirms that each step of the pathway is in place and functional within the microfluidic culture system.
Single-Islet Dynamic Stimulation
Next, we performed dynamic stimulations of the islets, fully taking advantage of the developed microfluidic setup that makes automatically switching between media of different compositions possible. For these experiments, we also added a cell-impermeant Zn2+-sensitive fluorophore (FluoZin-3) to the flowing culture media. Zn2+ measurement was used to indirectly evaluate the dynamics of insulin secretion, as Zn2+ is present within the intracellular insulin-containing granules undergoing exocytosis [30].
We analyzed single-islet response both upon switching between non-stimulatory (3 mM) and stimulatory (11 mM) glucose concentrations and in the presence of tolbutamide, an ATP-sensitive K+-channel blocker that induces insulin secretion even at non-stimulatory glucose concentrations [31]. As expected, tolbutamide produced a sudden increase of insulin secretion (Fig. 4a; Online Video 4) and of intracellular Ca2+ concentration (Fig. 4b).
Physiologic and pharmacologic validation of islet functionality. a The islet shown in the inset was dynamically perfused with different media, as indicated, all containing the FluoZin-3 Zn2+-sensitive fluorophore. This dye indicates Zn2+ secretion from the islet, as an indirect measurement of insulin secretion [21]. The plot shows changes of fluorescence intensity in the ROI shown in the inset. The false-colored images at the bottom show the spatial fluorescence intensity at different time points (see Video 4). b The islet shown in the inset was pre-loaded with FuraRed and dynamically perfused with different media, as indicated. Fluorescence intensity in the inset ROI was quantified in the plot that shows intracellular Ca2+ concentration
Altogether, these data demonstrate islet functionality within the microfluidic system, validate the detection method for analyzing single islets with high spatio-temporal resolution in response to stimulations, and give a demonstration of the high potential of this system for performing accurate dynamic studies.
Effects of Simvastatin on Insulin Secretion
In a previous study on MIN-6, a murine insulinoma cell line, simvastatin was shown to reduce insulin secretion by targeting ATP-sensitive K+-channels, voltage-gated Ca2+-channels, muscarinic M3 receptors, and GPR40 in a time span of approximately an hour [13]. On a shorter time scale (minutes), the same study demonstrated that simvastatin suppresses cytosolic Ca2+ regulation in response to both stimulatory glucose concentrations and tolbutamide in MIN-6 cells.
We used our validated microfluidic system to analyze short-term effects of simvastatin on the insulin secretion pathway using intact single pancreatic islets as a more physiologically relevant in vitro model.
After testing different concentrations of simvastatin from 1 to 10 μM (data not shown), we used a 1 μM concentration in all experiments, as the lowest concentration of drug that elicited an effect on islet function. We then analyzed the ability of simvastatin to modify insulin secretion by single islets in microfluidics. As shown in Fig. 5a and Online Video 5, simvastatin rapidly blocked insulin secretion even in the presence of a stimulatory glucose concentration (11 mM). The same inhibitory effect on secretion was observed independently of the sequence in which stimuli were applied (Fig. 5a, b). Such a rapid and reversible effect of simvastatin on single islets has never been shown before and was detected taking advantage of the small-scale transparent dynamic setup we developed.
Effects of simvastatin on glucose-induced insulin secretion. a Dynamics of insulin secretion, indirectly measured by FluoZin-3 fluorescence intensity, during islet perfusion with media of different compositions, as indicated. b Same as a, inverting the order of simvastatin stimulation at high-glucose concentration
To compare our data to those previously obtained with MIN-6 cells, we coupled the measurement of insulin secretion with that of cytosolic Ca2+. Unlike in Yaluri et al. [13], we found that cytosolic Ca2+ was not affected by simvastatin in single islets, despite its inhibition of insulin secretion at stimulatory glucose concentrations (Fig. 6a; Online Video 6). This suggests that simvastatin acts on the late stages of insulin granule trafficking, downstream of cytosolic Ca2+ regulation. However, when tolbutamide was concomitantly used to induce insulin secretion, simvastatin did not prevent a cytosolic Ca2+ increase and insulin secretion, at both low (3 mM) and high (11 mM) glucose concentrations (Fig. 6b). Similarly, islet treatment with simvastatin, prior to addition of tolbutamide, did not prevent the rise in cytosolic Ca2+ concentration and insulin secretion (Fig. 6c).
Discussion
In this study, we developed and validated a microfluidic platform optimized for functional analysis of single pancreatic islets by live confocal and two-photon imaging. Microfluidic systems are increasingly used to study physiological phenomena in vitro, with several advantages [32, 33]. The possibility to analyze a single islet, the fundamental unit of endocrine pancreas, preserves intercellular interactions within the islet and avoids the confounding complexity of whole animal physiology and inter-islet communication as in organo-culture. By using a miniaturized multichannel system we could remotely control the switch of the perfusion medium entering the microfluidic chamber with a resolution of seconds and analyze live how a single islet responds to different exogenous stimuli. In vivo imaging of the endocrine pancreas is the most suitable physiological model to assess insulin secretion. However, this is poorly standardized, highly invasive, and laborious. In particular, it is difficult to deliver precisely localized stimuli, while dynamically visualizing β-cell response. Ex vivo intact islets are fully functional units, whereas β-cell lines are immortalized and often not pure: the commonly used MIN6 cell line contains a mix of pancreatic endocrine cells [34]. Furthermore, primary islets can be quickly isolated and immediately used for imaging, whereas β-cell lines are difficult to maintain in culture (Table 1).
Using confocal and two-photon imaging, we showed normal functionality of single islets within the microfluidic device in terms of synchronized membrane voltage and intracellular Ca2+ regulation, as well as glucose- and tolbutamide-stimulated insulin secretion.
As a clinically relevant application of this setup, we explored the effects of the HMG-CoA reductase inhibitor, simvastatin, on Ca2+ dynamics and insulin secretion. Several reports suggest that statins may increase the risk of developing diabetes by acting on insulin secretion, insulin resistance, or both [11, 12, 35, 36]. However, whether statins truly inhibit insulin secretion is unclear.
Pooling together five murine islets, Zúñiga-Hertz et al. demonstrate that simvastatin interferes with isoprenylation of proteins involved in insulin granule exocytosis on a 2-h time scale and with cholesterol biosynthesis on a 24-h time scale [14]. Shorter time effects of simvastatin on the GSIS pathway have only been demonstrated in vitro using a murine β-cell line, MIN-6, and only as for its action on cytosolic Ca2+ dynamics [13].
Here, we extended the results previously obtained with MIN-6 cells to single, freshly isolated murine islets. While Yaluri et al. [13] found that simvastatin affects Ca2+ regulation, in our results single islet cytosolic Ca2+ is not perturbed by simvastatin. Moreover, for the first time, we were able to demonstrate that simvastatin affects insulin secretion of single islets, a result previously shown only by pooling more islets together, a less physiological model because of the in vivo islet–islet coordination [37], and analyzing insulin secretion only on much longer time scales. The rapidity of the simvastatin effect on insulin secretion suggests its involvement in regulatory mechanisms other than cholesterol biosynthesis and post-translational modifications, which require more time to occur.
Ultimately, we showed that single islets respond to simvastatin acutely and reversibly, blocking the normal insulin secretion induced by high glucose. As there is no effect of simvastatin on Ca2+ intracellular concentration and dynamics, we suggest that the inhibitory effect on insulin secretion may be exerted at the levels of insulin granule trafficking. Moreover, tolbutamide can effectively counteract the inhibitory effect of simvastatin on insulin secretion, suggesting that simvastatin's action is reversible not only by its withdrawal, but also by Ca2+ overload and membrane depolarization. Reversibility of the effect has clinical implications and likely reflects the relatively benign effect of statins on diabetes onset or progression. Limitations of this study are inherent to the short time course of observation and the lack of a dose-dependent response. Table 2 highlights the advancement over the state of the art made by our study.
Conclusion
In conclusion, we report that simvastatin rapidly and reversibly impairs insulin release from intact islets ex vivo. Further work is needed to understand the underlying mechanisms of action of simvastatin at the second-to-minute scale. The microfluidic and imaging techniques herein described will be suitable to dissect the molecular and electrophysiological processes underlying this statin effect. The possibility to use concomitant treatments will help to clarify whether the effect of simvastatin can be counteracted by other glucose-lowering agents with mechanisms of action different from sulfonylureas, such as GLP-1 receptor agonists or even SLGT2 inhibitors.
References
Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet Lond Engl. 2016;S0140-6736-5.
Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;S0828-282-2.
Dong N, Xie Z, Dai J, Wang W, Sun R, Zhan Y, et al. Statin-induced improvements in vulnerable plaques are attenuated in poorly controlled diabetic patients with coronary atherosclerosis disease: a serial optical coherence tomography analysis. Acta Diabetol. 2016. doi:10.1007/s00592-016-0902-9.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2016 Executive summary. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2016;22:84–113.
Chamberlain JJ, Rhinehart AS, Shaefer CF, Neuman A. Diagnosis and management of diabetes: synopsis of the 2016 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2016;164:542–52.
Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HAW, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet Lond Engl. 2004;364:685–96.
Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JEL, Shah T, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet Lond Engl. 2015;385:351–61.
Navarese EP, Buffon A, Andreotti F, Kozinski M, Welton N, Fabiszak T, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123–30.
Preiss D, Seshasai SRK, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–64.
Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet Lond Engl. 2010;375:735–42.
Erqou S, Lee CC, Adler AI. Statins and glycaemic control in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. 2014;57:2444–52.
Cederberg H, Stančáková A, Yaluri N, Modi S, Kuusisto J, Laakso M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow-up study of the METSIM cohort. Diabetologia. 2015;58:1109–17.
Yaluri N, Modi S, López Rodríguez M, Stančáková A, Kuusisto J, Kokkola T, et al. simvastatin impairs insulin secretion by multiple mechanisms in MIN6 cells. PLoS One. 2015;10:e0142902.
Zúñiga-Hertz JP, Rebelato E, Kassan A, Khalifa AM, Ali SS, Patel HH, et al. Distinct pathways of cholesterol biosynthesis impact on insulin secretion. J Endocrinol. 2015;224:75–84.
Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet Lond. Engl. 2012;380:565–71.
Blackburn DF, Chow JY, Smith AD. Statin use and incident diabetes explained by bias rather than biology. Can J Cardiol. 2015;31:966–9.
Robinson JG. Statins and diabetes risk: how real is it and what are the mechanisms? Curr Opin Lipidol. 2015;26:228–35.
Wang Y, Mendoza-Elias J, McGarrigle J, Nourmohammadzadeh M, Wang Q, Li Z, et al. Application of microfluidic technology for studying islet physiology and pathophysiology. Micro Nanosyst. 2013;5:216–23.
Gagliano O, Elvassore N, Luni C. Microfluidic technology enhances the potential of human pluripotent stem cells. Biochem Biophys Res Commun. 2016;473:683–7.
Benninger RKP, Hutchens T, Head WS, McCaughey MJ, Zhang M, Le Marchand SJ, et al. Intrinsic islet heterogeneity and gap junction coupling determine spatiotemporal Ca2+ wave dynamics. Biophys J. 2014;107:2723–33.
Lomasney AR, Yi L, Roper MG. Simultaneous monitoring of insulin and islet amyloid polypeptide secretion from islets of langerhans on a microfluidic device. Anal Chem. 2013;85:7919–25.
Zambon A, Zoso A, Luni C, Frommer WB, Elvassore N. Determination of glucose flux in live myoblasts by microfluidic nanosensing and mathematical modeling. Integr Biol. 2014;6:277–88.
Dishinger JF, Reid KR, Kennedy RT. Quantitative monitoring of insulin secretion from single islets of langerhans in parallel on a microfluidic chip. Anal Chem. 2009;81:3119–27.
Stull ND, Breite A, McCarthy R, Tersey SA, Mirmira RG. Mouse islet of Langerhans isolation using a combination of purified collagenase and neutral protease. J Vis Exp. 2012. doi:10.3791/4137.
Miller EW, Lin JY, Frady EP, Steinbach PA, Kristan WB, Tsien RY. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc Natl Acad Sci USA. 2012;109:2114–9.
Ciubotaru CD, Bastianello S, Beltramello M, Pozzan T, Mammano F. Multi-modal imaging of cytosolic and mitochondrial Ca2+. In: Adlassnig K-P, Bracale M, editors. Anaheim: ACTA Press; 2005. p. 99–102.
Kurebayashi N, Harkins AB, Baylor SM. Use of fura red as an intracellular calcium indicator in frog skeletal muscle fibers. Biophys J. 1993;64:1934–60.
Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52:739–51.
Santos RM, Rosario LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflugers Arch. 1991;418:417–22.
Dunn MF. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer—a review. Biometals Int J Role Met Ions BiolBiochem Med. 2005;18:295–303.
Trube G, Rorsman P, Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986;407:493–9.
Salieb-Beugelaar GB, Simone G, Arora A, Philippi A, Manz A. Latest developments in microfluidic cell biology and analysis systems. Anal Chem. 2010;82:4848–64.
Wang D, Bodovitz S. Single cell analysis: the new frontier in “omics”. Trends Biotechnol. 2010;28:281–90.
Nakashima K, Kanda Y, Hirokawa Y, Kawasaki F, Matsuki M, Kaku K. MIN6 is not a pure beta cell line but a mixed cell line with other pancreatic endocrine hormones. Endocr J. 2009;56:45–53.
Livingstone SJ, Looker HC, Akbar T, Betteridge DJ, Durrington PN, Hitman GA, et al. Effect of atorvastatin on glycaemia progression in patients with diabetes: an analysis from the Collaborative Atorvastatin in Diabetes Trial (CARDS). Diabetologia. 2016;59:299–306.
Swerdlow DI, Sattar N. A dysglycaemic effect of statins in diabetes: relevance to clinical practice? Diabetologia. 2014;57:2433–5.
Pedersen MG, Bertram R, Sherman A. Intra- and inter-islet synchronization of metabolically driven insulin secretion. Biophys J. 2005;89:107–19.
Acknowledgments
This work was supported by a grant from the University of Padova to AA and FM (Progetto Strategico DYCENDI) and by the EASD Foundation to NE. All named authors meet the International Committee of Medical Journal Editors (ICJME) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The article processing charges were funded by the DYCENDI project.
Disclosures
Valentina Scattolini, Camilla Luni, Alessandro Zambon, Silvia Galvanin, Onelia Gagliano, Catalin Dacian Ciubotaru, Angelo Avogaro, Fabio Mammano, Nicola Elvassore, and Gian Paolo Fadini have nothing to disclose.
Compliance with Ethics Guidelines
The institutional review board of the Venetian Institute of Molecular Medicine approved the study. The protocol followed the Guide for the care and use of laboratory animals, Eighth edition (2011) (http://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf). All institutional and national guidelines for the care and use of laboratory animals were followed.
Data Availability
The datasets used during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Enhanced content
The online video files are available to view at http://www.medengine.com/Redeem/6317F060735F7A3B.
An erratum to this article is available at http://dx.doi.org/10.1007/s13300-016-0216-5.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Scattolini, V., Luni, C., Zambon, A. et al. Simvastatin Rapidly and Reversibly Inhibits Insulin Secretion in Intact Single-Islet Cultures. Diabetes Ther 7, 679–693 (2016). https://doi.org/10.1007/s13300-016-0210-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-016-0210-y