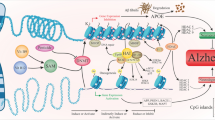
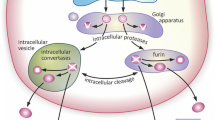
Abstract
Memories are believed to be represented by facilitated synaptic transmission of electrical signals in neuronal networks. The ability to acquire new memories or to change old memory content results from the plastic properties of the brain. Molecular changes in synaptic plasticity of neuronal networks are considered to be the cellular correlates of learning and memory, and the neurotrophin brain-derived neurotropic factor (BDNF) plays an important role in these processes. This neurotrophic factor coordinates a multitude of biological functions. In addition to its role in neuronal plasticity processes, such as long-term potentiation of synaptic transmission, the protein regulates the differentiation of neuronal precursor cells, synaptogenesis, and neuronal survival. Cellular processes like BDNF protein processing, anterograde and retrograde transport, as well as exocytosis and endocytosis of BDNF vesicles are necessary to enable the protein to fulfill its neuroprotective and plasticity-related functions in its target areas. Therefore, deficits in one of these functions, resulting in a reduction or a lack of BDNF supply, can result in dysfunctional or reduced synaptic plasticity in virtually every brain area. Since cognitive processes and mental health require the intact formation and modification of memory traces, a change in BDNF turnover is considered as a contributing factor to a number of neurodegenerative and psychological disorders. This review summarizes the current knowledge regarding the connection between BDNF, its role in synaptic plasticity and its role in brain.






Similar content being viewed by others
References
Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356
Lewin GR, Barde YA (1996) Physiology of the neurotrophins. Annu Rev Neurosci 19:289–317
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14:7–23
Lessmann V, Brigadski T (2009) Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res 65:11–22
Matsumoto T, Rauskolb S, Polack M et al (2008) Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci 11:131–133
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 361:1545–1564
Zagrebelsky M, Korte M (2014) Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology 76 Pt C:628–638
Goodman LJ, Valverde J, Lim F et al (1996) Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci 7:222–238
Edelmann E, Lessmann V, Brigadski T (2014) Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology 76 Pt C:610–627
Dieni S, Matsumoto T, Dekkers M et al (2012) BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol 196:775–788
Tongiorgi E (2008) Activity-dependent expression of brain-derived neurotrophic factor in dendrites: facts and open questions. Neurosci Res 61:335–346
English CN, Vigers AJ, Jones KR (2012) Genetic evidence that brain-derived neurotrophic factor mediates competitive interactions between individual cortical neurons 2. Proc Natl Acad Sci U S A 109:19456–19461
Kohara K, Yasuda H, Huang Y et al (2007) A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci 27:7234–7244
Gottmann K, Mittmann T, Lessmann V (2009) BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res 199:203–234
Lessmann V, Gottmann K, Malcangio M (2003) Neurotrophin secretion: current facts and future prospects. Prog Neurobiol 69:341–374
Balkowiec A, Katz DM (2002) Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci 22:10399–10407
Kuczewski N, Porcher C, Ferrand N et al (2008) Backpropagating action potentials trigger dendritic release of BDNF during spontaneous network activity. J Neurosci 28:7013–7023
Matsuda N, Lu H, Fukata Y et al (2009) Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci 29:14185–14198
Kolarow R, Brigadski T, Lessmann V (2007) Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J Neurosci 27:10350–10364
Brigadski T, Hartmann M, Lessmann V (2005) Differential vesicular targeting and time course of synaptic secretion of the mammalian neurotrophins. J Neurosci 25:7601–7614
Hartmann M, Heumann R, Lessmann V (2001) Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J 20:5887–5897
Angleson JK, Cochilla AJ, Kilic G et al (1999) Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nat Neurosci 2:440–446
Barg S, Olofsson CS, Schriever-Abeln J et al (2002) Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells. Neuron 33:287–299
Bonhoeffer T (1996) Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol 6:119–126
Abidin I, Kohler T, Weiler E et al (2006) Reduced presynaptic efficiency of excitatory synaptic transmission impairs LTP in the visual cortex of BDNF-heterozygous mice. Eur J Neurosci 24:3519–3531
Figurov A, Pozzo-Miller LD, Olafsson P et al (1996) Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381:706–709
Itami C, Kimura F, Kohno T et al (2003) Brain-derived neurotrophic factor-dependent unmasking of “silent” synapses in the developing mouse barrel cortex. Proc Natl Acad Sci U S A 100:13069–13074
Kang H, Welcher AA, Shelton D, Schuman EM (1997) Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron 19:653–664
Korte M, Carroll P, Wolf E et al (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A 92:8856–8860
Meis S, Endres T, Lessmann V (2012) Postsynaptic BDNF signalling regulates long-term potentiation at thalamo-amygdala afferents. J Physiol 590:193–208
Schildt S, Endres T, Lessmann V, Edelmann E (2013) Acute and chronic interference with BDNF/TrkB-signaling impair LTP selectively at mossy fiber synapses in the CA3 region of mouse hippocampus. Neuropharmacology 71:247–254
Kossel AH, Cambridge SB, Wagner U, Bonhoeffer T (2001) A caged Ab reveals an immediate/instructive effect of BDNF during hippocampal synaptic potentiation. Proc Natl Acad Sci U S A 98:14702–14707
Zakharenko SS, Patterson SL, Dragatsis I et al (2003) Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron 39:975–990
Gartner A, Polnau DG, Staiger V et al (2006) Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cgamma signaling. J Neurosci 26:3496–3504
Korte M, Bonhoeffer T (1997) Activity-dependent synaptic plasticity: a new face of action for neurotrophins. Mol Psychiatry 2:197–199
Korte M, Kang H, Bonhoeffer T, Schuman E (1998) A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology 37:553–559
Tanaka J, Horiike Y, Matsuzaki M et al (2008) Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 319:1683–1687
Tyler WJ, Pozzo-Miller L (2003) Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol 553:497–509
Abidin I, Eysel UT, Lessmann V, Mittmann T (2008) Impaired GABAergic inhibition in the visual cortex of brain-derived neurotrophic factor heterozygous knockout mice. J Physiol 586:1885–1901
Gubellini P, Ben-Ari Y, Gaiarsa JL (2005) Endogenous neurotrophins are required for the induction of GABAergic long-term potentiation in the neonatal rat hippocampus. J Neurosci 25:5796–5802
Kuczewski N, Langlois A, Fiorentino H et al (2008) Spontaneous glutamatergic activity induces a BDNF-dependent potentiation of GABAergic synapses in the newborn rat hippocampus. J Physiol 586:5119–5128
Mohajerani MH, Sivakumaran S, Zacchi P et al (2007) Correlated network activity enhances synaptic efficacy via BDNF and the ERK pathway at immature CA3 CA1 connections in the hippocampus. Proc Natl Acad Sci U S A 104:13176–13181
Sivakumaran S, Mohajerani MH, Cherubini E (2009) At immature mossy-fiber-CA3 synapses, correlated presynaptic and postsynaptic activity persistently enhances GABA release and network excitability via BDNF and cAMP-dependent PKA. J Neurosci 29:2637–2647
Holsinger RM, Schnarr J, Henry P et al (2000) Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer’s disease. Brain Res Mol Brain Res 76:347–354
Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S (2008) New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev 59:201–220
Pan W, Banks WA, Fasold MB et al (1998) Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 37:1553–1561
Krabbe KS, Nielsen AR, Krogh-Madsen R et al (2007) Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 50:431–438
Rasmussen P, Brassard P, Adser H et al (2009) Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol 94:1062–1069
Kerschensteiner M, Gallmeier E, Behrens L et al (1999) Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 189:865–870
Fujimura H, Altar CA, Chen R et al (2002) Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost 87:728–734
Karege F, Bondolfi G, Gervasoni N et al (2005) Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry 57:1068–1072
Conner JM, Yan Q, Varon S (1996) Distribution of brain-derived neurotrophic factor in the rat pituitary gland. Neuroreport 7:1937–1940
Nakahashi T, Fujimura H, Altar CA et al (2000) Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett 470:113–117
Castren E, Rantamaki T (2010) The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol 70:289–297
Laske C, Stellos K, Hoffmann N et al (2011) Higher BDNF serum levels predict slower cognitive decline in Alzheimer’s disease patients. Int J Neuropsychopharmacol 14:399–404
Egan MF, Kojima M, Callicott JH et al (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269
Intlekofer KA, Cotman CW (2013) Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis 57:47–55
Acknowledgments
The authors thank Petra Lichtenecker for the secretion data in Fig. 3 as well as Elke Edelmann and Thomas Endres (all members of the Institute of Physiology in Magdeburg) for a number of discussions, which have essentially contributed to the contents of this review article. The English translation of this article was kindly provided by Petra Lichtenecker and Angela M. Cole.
Our work is supported by the DFG (SFB 779, TP B6; LE 1020/2-1), the federal state of Saxony-Anhalt, as well as the “Europäischer Fond für regionale Entwicklung” (EFRE—Projekt: Center of Behavioral Brain Sciences (CBBS)).
Compliance with ethical guidelines
Conflict of interest. T. Brigadski and Leßmann state that there are no conflicts of interest.
The accompanying manuscript does not include studies on humans or animals.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Brigadski, T., Leßmann, V. BDNF: a regulator of learning and memory processes with clinical potential. e-Neuroforum 5, 1–11 (2014). https://doi.org/10.1007/s13295-014-0053-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13295-014-0053-9