Abstract
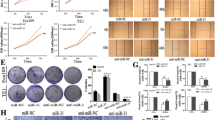
Accumulated evidence suggests that miR-106b played a key role in the promotion of the metastases of cancer; however, little is known about miR-106b in esophageal squamous cell carcinoma (ESCC). To investigate expression level of miR-106b in ESCC tissues, quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect miR-106b expression in 35 Kazakh’s ESCC and paired normal adjacent tissues (NATs). To evaluate the role mediated by miR-106b in the proliferation, migration, and invasion, MTT, wound healing, and transwell assays were employed, respectively. Luciferase reporter assay was used to identify the downstream target through miR-106b. To understand the regulation between miR-106b and Smad 7, qRT-PCR and western blot were performed. The present study showed that miR-106b was pronouncedly upregulated in ESCC relative to paired NAT and that upregulated miR-106b was significantly associated with lymph node metastases. MiR-106b was found to be able to promote proliferation, migration, and invasion of ESCC cells in vitro. Smad 7 was confirmed as a downstream target of miR-106b in our experimental setting. Smad 7 was remarkably downregulated in ESCC compared with paired NAT. In addition, upregulation of miR-106b can promote epithelial mesenchymal transition (EMT) in ESCC cell in vitro. Our results indicated that miR-106b can promote migration and invasion of ESCC cells through enhancing EMT process via downregulation of Smad 7, suggesting that miR-106b can be a potential molecular phenotype in ESCC metastases.






Similar content being viewed by others
References
Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: risk factors, screening and endoscopic treatment in western and eastern countries. World J Gastroenterol. 2015;21:7933–43.
Tang WR, Chen ZJ, Lin K, Su M, Au WW. Development of esophageal cancer in Chaoshan region, China: association with environmental, genetic and cultural factors. Int J Hyg Environ Health. 2015;218:12–8.
Zheng ST, Vuitton L, Sheyhidin I, Vuitton DA, Zhang YM, Lu XM. North western China: a place to learn more on oesophageal cancer. I. Behavioural and environmental risk factors. Eur J Gastroenterol Hepatol. 2010;22(8):917–25.
Cho JW, Choi SC, Jang JY, Shin SK, Choi KD, Lee JH, et al. Lymph node metastases in esophageal carcinoma: an endoscopist’s view. Clinical endoscopy. 2014;47:523–9.
Park JH, Shin C. MicroRNA-directed cleavage of targets: mechanism and experimental approaches. BMB Rep. 2014;47:417–23.
Iorio MV, Croce CM. Causes and consequences of microRNA dysregulation. Cancer J. 2012;18:215–22.
Petrocca F, Vecchione A, Croce CM. Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor beta signaling. Cancer Res. 2008;68:8191–4.
Xu Y, Wang K, Gao W, Zhang C, Huang F, Wen S, et al. MicroRNA-106b regulates the tumor suppressor RUNX3 in laryngeal carcinoma cells. FEBS Lett. 2013;587:3166–74.
Yang TS, Yang XH, Chen X, Wang XD, Hua J, Zhou DL, et al. MicroRNA-106b in cancer-associated fibroblasts from gastric cancer promotes cell migration and invasion by targeting PTEN. FEBS Lett. 2014;588:2162–9.
Li Y, Tan W, Neo TW, Aung MO, Wasser S, Lim SG, et al. Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci. 2009;100:1234–42.
Smith AL, Iwanaga R, Drasin DJ, Micalizzi DS, Vartuli RL, Tan AC, et al. The miR-106b-25 cluster targets Smad 7, activates TGF-beta signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene. 2012;31:5162–71.
Yang J, Zhou F, Xu T, Deng H, Ge YY, Zhang C, et al. Analysis of sequence variations in 59 microRNAs in hepatocellular carcinomas. Mutat Res. 2008;638:205–9.
Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F, et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68:6162–70.
Pichiorri F, Suh SS, Ladetto M, Kuehl M, Palumbo T, Drandi D, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc of the Nati Acad of Sci of the United States of America. 2008;105(35):12885–90.
Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni L, et al. MicroRNA profiling of human gastric cancer. Mol Med Rep. 2009;2:963–70.
Kan T, Meltzer SJ. MicroRNAs in Barrett’s esophagus and esophageal adenocarcinoma. Curr Opin Pharmacol. 2009;9:727–32.
Hui AB, Lenarduzzi M, Krushel T, Waldron L, Pintilie M, Shi W, et al. Comprehensive MicroRNA profiling for head and neck squamous cell carcinomas. Clin Cancer Res. 2010;16(4):1129–39.
Kan T, Sato F, Ito T, Matsumura N, David S, Cheng Y, et al. The miR-106b-25 polycistron, activated by genomic amplification, functions as an oncogene by suppressing p21 and Bim. Gastroenterology. 2009;136:1689–700.
Gu J, Wang Y, Wu X. MicroRNA in the pathogenesis and prognosis of esophageal cancer. Curr Pharm Des. 2013;19:1292–300.
Zhang GJ, Li JS, Zhou H, Xiao HX, Li Y, Zhou T. MicroRNA-106b promotes colorectal cancer cell migration and invasion by directly targeting DLC1. Journal of experimental & clinical cancer research: CR. 2015;34:73.
Xiang W, He J, Huang C, Chen L, Tao D, Wu X, et al. miR-106b-5p targets tumor suppressor gene SETD2 to inactive its function in clear cell renal cell carcinoma. Oncotarget. 2015;6:4066–79.
Li KK, Xia T, Ma FM, Zhang R, Mao Y, Wang Y, et al. miR-106b is overexpressed in medulloblastomas and interacts directly with PTEN. Neuropathol Appl Neurobiol. 2015;41:145–64.
Zhou Y, Hu Y, Yang M, Jat P, Li K, Lombardo Y, et al. The miR-106b~25 cluster promotes bypass of doxorubicin-induced senescence and increase in motility and invasion by targeting the E-cadherin transcriptional activator EP300. Cell Death Differ. 2014;21:462–74.
Zheng L, Zhang Y, Lin S, Sun A, Chen R, Ding Y, et al. Down-regulation of miR-106b induces epithelial-mesenchymal transition but suppresses metastatic colonization by targeting Prrx1 in colorectal cancer. Int J Clin Exp Patho. 2015;8:10534–44.
Gong C, Qu S, Liu B, Pan S, Jiao Y, Nie Y, et al. MiR-106b expression determines the proliferation paradox of TGF-beta in breast cancer cells. Oncogene. 2015;34:84–93.
Li F, Liu J, Li S. MicorRNA 106b approximately 25 cluster and gastric cancer. Surg Oncol. 2013;22:e7–10.
Yau WL, Lam CS, Ng L, Chow AK, Chan ST, Chan JY, et al. Over-expression of miR-106b promotes cell migration and metastasis in hepatocellular carcinoma by activating epithelial-mesenchymal transition process. PLoS One. 2013;8:e57882.
Dong P, Kaneuchi M, Watari H, Sudo S, Sakuragi N. MicroRNA-106b modulates epithelial-mesenchymal transition by targeting TWIST1 in invasive endometrial cancer cell lines. Mol Carcinog. 2014;53:349–59.
Yu D, Shin HS, Lee YS, Lee YC. miR-106b modulates cancer stem cell characteristics through TGF-beta/Smad signaling in CD44-positive gastric cancer cells. Lab Investig. 2014;94(12):1370–81.
Qu MH, Han C, Srivastava AK, Cui T, Zou N, Gao ZQ, et al. miR-93 promotes TGF-beta-induced epithelial-to-mesenchymal transition through downregulation of NEDD4L in lung cancer cells. Tumour bio. 2016;37(4):5645–51.
Morata-Tarifa C, Jimenez G, Garcia MA, Entrena JM, Grinan-Lison C, Aguilera M, et al. Low adherent cancer cell subpopulations are enriched in tumorigenic and metastatic epithelial-to-mesenchymal transition-induced cancer stem-like cells. Scientific reports. 2016;6:18772.
Li Y, Chen D, Su Z, Li Y, Liu J, Jin L, et al. MicroRNA106b functions as an oncogene in renal cell carcinoma by affecting cell proliferation, migration and apoptosis. Mol Med Rep. 2016;13:1420–6.
Zheng R, Pan L, Gao J, Ye X, Chen L, Zhang X, et al. Prognostic value of miR-106b expression in breast cancer patients. J Surg Res. 2015;195:158–65.
Acknowledgments
This work was supported by the Natural Foundation of Natural China (nos. 81160303, 81260359, 81201891, U1303321), Major Science and Technology Projects of the Xinjiang Uygur Autonomous Region (no. 201430123-1), Xinjiang Key Laboratory of Major Diseases Grant (no. 2014Y3), and Postgraduate Research Innovation Project of Xinjiang Uygur Autonomous Region (no. XJGRI2015063).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Fang Dai and Tao Liu contributed equally to this work.
Electronic supplementary material
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Dai, F., Liu, T., Zheng, S. et al. MiR-106b promotes migration and invasion through enhancing EMT via downregulation of Smad 7 in Kazakh’s esophageal squamous cell carcinoma. Tumor Biol. 37, 14595–14604 (2016). https://doi.org/10.1007/s13277-016-5338-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5338-x