Abstract
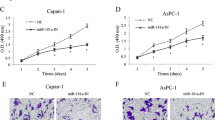
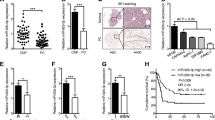
Pancreatic carcinoma is one of the most malignant human cancers. In this study, we intended to explore the molecular functional of microRNA-127 (miR-127) in regulating pancreatic cancer development both in vitro and in vivo. Quantitative real-time PCR (qRT-PCR) was performed to evaluate endogenous miR-127 expression in in vitro pancreatic cancer cell lines and in vivo clinical samples of pancreatic carcinoma. Lentiviral technology was applied to overexpress miR-127 in capan-1 and PANC-1 cells. Pancreatic cancer proliferation, cell-cycle progression, and invasion were assessed in vitro, and capan-1-derived tumorigenicity was evaluated in vivo. Dual-luciferase reporter assay and qRT-PCR were performed to assess the downstream target gene of miR-127 in pancreatic cancer, human Bcl-2-associated athanogene 5 (BAG5). BAG5 was subsequently upregulated in miR-127-overexpressed capan-1 and PANC-1 cells to evaluate its effect on pancreatic cancer progression. MiR-127 was preferentially downregulated in both pancreatic carcinoma cell lines and human pancreatic tumors. In lentivirus-infected capan-1 and PANC-1 cells, miR-127 overexpression significantly inhibited cancer progression, cell-cycle transition and invasion in vitro, as well as tumorigenicity in vivo. Human BAG5 was confirmed to be the downstream target of miR-127 in pancreatic cancer. Forced overexpression of BAG5 in capan-1 and PANC-1 cells reversed the tumor-suppressing effect of miR-127 on cancer development. MiR-127 is downregulated and acting as a tumor suppressor in pancreatic carcinoma. The functional regulation of miR-127 in pancreatic carcinoma is very likely through the inverse correlation of its downstream target gene of BAG5.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–26.
Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. World J Gastroenterol. 2015;21:3157–65.
Mohammed S, Van Buren 2nd G, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol. 2014;20:9354–60.
Puleo F, Marechal R, Demetter P, Bali MA, Calomme A, Closset J, Bachet JB, Deviere J, Van Laethem JL. New challenges in perioperative management of pancreatic cancer. World J Gastroenterol. 2015;21:2281–93.
Afonso-Grunz F, Muller S. Principles of miRNA-mRNA interactions: beyond sequence complementarity. Cell Mol Life Sci. 2015;72:3127–41.
Takasaki S. Roles of microRNAs in cancers and development. Methods Mol Biol. 2015;1218:375–413.
Zhang R, B S. Small but influential: the role of microRNAs on gene regulatory network and 3′UTR evolution. J Genet Genomics. 2009;36:1–6.
Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8.
Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–79.
Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12.
Lynam-Lennon N, Maher SG, Reynolds JV. The roles of microRNA in cancer and apoptosis. Biol Rev Camb Philos Soc. 2009;84:55–71.
Babashah S, Soleimani M. The oncogenic and tumour suppressive roles of microRNAs in cancer and apoptosis. Eur J Cancer. 2011;47:1127–37.
Gayral M, Jo S, Hanoun N, Vignolle-Vidoni A, Lulka H, Delpu Y, Meulle A, Dufresne M, Humeau M, Chalret du Rieu M, Bournet B, Selves J, Guimbaud R, Carrere N, Buscail L, Torrisani J, Cordelier P. MicroRNAs as emerging biomarkers and therapeutic targets for pancreatic cancer. World J Gastroenterol. 2014;20:11199–209.
Humeau M, Torrisani J, Cordelier P. miRNA in clinical practice: pancreatic cancer. Clin Biochem. 2013;46:933–6.
Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171–6.
Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D, Fearon ER, Lawrence TS, Xu L. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816.
Correa-Medina M, Bravo-Egana V, Rosero S, Ricordi C, Edlund H, Diez J, Pastori RL. MicroRNA miR-7 is preferentially expressed in endocrine cells of the developing and adult human pancreas. Gene Expr Patterns. 2009;9:193–9.
Yu J, Li A, Hong SM, Hruban RH, Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Res. 2012;18:981–92.
Doong H, Vrailas A, Kohn EC. What's in the ‘BAG’?—a functional domain analysis of the BAG-family proteins. Cancer Lett. 2002;188:25–32.
Bruchmann A, Roller C, Walther TV, Schafer G, Lehmusvaara S, Visakorpi T, Klocker H, Cato AC, Maddalo D. Bcl-2 associated athanogene 5 (Bag5) is overexpressed in prostate cancer and inhibits ER-stress induced apoptosis. BMC Cancer. 2013;13:96.
Lai XL, Huang YH, Li YS, Li GN, Wang LP, Sun R, Ma YS, Feng SY, Chang ZY, Wang XH, Fu D, Han X, Cong XL, Li WP. Differential expression profiling of microRNAs in para-carcinoma, carcinoma and relapse human pancreatic cancer. Clin Transl Oncol. 2015;17:398–408.
Chen J, Wang M, Guo M, Xie Y, Cong YS. miR-127 regulates cell proliferation and senescence by targeting BCL6. PLoS One. 2013;8:e80266.
Guo LH, Li H, Wang F, Yu J, He JS. The tumor suppressor roles of miR-433 and miR-127 in gastric cancer. Int J Mol Sci. 2013;14:14171–84.
Ying Z, Haiyan G, Haidong G. BAG5 regulates PTEN stability in MCF-7 cell line. BMB Rep. 2013;46:490–4.
Acknowledgment
This work is supported by Grants from the National Natural Science Foundation of China (No.30872510, 81272534, 81260349, 81370569); and the Natural Science Foundation of Songjiang District (No.15SJGG23) and Key Program of Medical Specialty Construction in Shanghai City (ZK2015B20).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
Ethical approval for this study was granted by the Clinical Research and Ethic Committee at the Fifth People’s Hospital of Shanghai and Songjiang Hospital Affiliated to The First People’s Hospital of Shanghai Jiaotong University in Shanghai, China. Consent forms were signed by all participating patients. All protocols were performed in accordance with the Declaration of Helsinki and the guidelines for good clinical practice in China.
Conflicts of interest
None
Additional information
Yuan Yu and Lei Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, Y., Liu, L., Ma, R. et al. MicroRNA-127 is aberrantly downregulated and acted as a functional tumor suppressor in human pancreatic cancer. Tumor Biol. 37, 14249–14257 (2016). https://doi.org/10.1007/s13277-016-5270-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5270-0