Abstract
Radiotherapy is widely used for advanced rectal tumors. However, tumor recurrence after this treatment tends to be more aggressive and is associated with a poor prognosis. Uncovering the molecular mechanism that controls this recurrence is essential for developing new therapeutic applications. In the present study, we demonstrated that radiation increases the EphA4 activation level of the survivor progeny of colorectal cancer cells submitted to this treatment and that such activation promoted the internalization of a complex E-cadherin-EphA4, inducing cell–cell adhesion disruption. Moreover, EphA4 knockdown in the progeny of irradiated cells reduced the migratory and invasive potentials and metalloprotease activity induced by irradiation. Finally, we demonstrated that the cell migration and invasion potential were regulated by AKT and ERK1/2 signaling, with the ERK1/2 activity being dependent on EphA4. In summary, our study demonstrates that these signaling pathways could be responsible for the therapeutic failure, thereby promoting local invasion and metastasis in rectal cancer after radiotherapy. We also postulate that EphA4 is a potential therapeutic target for colorectal cancer treatment.







Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Instituto Nacional de Câncer José Alencar Gomes da Silva; Ministério da Saúde. Estimativa 2014. 2013; Rio de Janeiro.
Glynne-Jones R, Hadaki M, Harrison M. The status of targeted agents in the setting of neoadjuvant radiation therapy in locally advanced rectal cancers. J Gastrointest Oncol. 2013;4(3):264–84.
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, German Rectal Cancer Study Group, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358(9290):1291–304.
Madani I, de Neve W, Mareel M. Does ionizing radiation stimulate cancer invasion and metastasis? Bull Cancer. 2008;95(3):292–300.
Vicini F, Kestin L, Huang R, Martinez A. Does local recurrence affect the rate of distant metastases and survival in patients with early-stage breast carcinoma treated with breast-conserving therapy? Cancer. 2003;97(4):910–9.
van den Brink M, Stiggelbout AM, van den Hout WB, Kievit J, Klein Kranenbarg E, et al. Clinical nature and prognosis of locally recurrent rectal cancer after total mesorectal excision with or without preoperative radiotherapy. J Clin Oncol. 2004;22:3958–64.
Prise K, Schettino G, Folkard M. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6(7):520–8.
Alexander S, Friedl P. Cancer invasion and resistance: interconnected processes of disease progression and therapy failure. Trends Mol Med. 2012;18:13–26.
Yacoub A, Miller A, Caron RW, Qiao L, Curiel DA, et al. Radiotherapy-induced signal transduction. Endocr Relat Cancer. 2006;13:S99–114.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, RECORD‐1 Study Group, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–65.
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–23.
Kullarnder K, Klein R. Mechanisms and functions of Eph and ephrin signaling. Nat Rev Mol Cell Biol. 2002;7:475–86.
Fox BP, Kandpal RP. Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem Biophys Res Commun. 2004;318(4):882–92.
Fukai J, Yokote H, Yamanaka R, Arao T, Nishio K, Itakura T. EphA4 promotes cell proliferation and migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol Cancer Ther. 2008;7(9):2768–78.
Yan Y, Luo YC, Wan HY, Wang J, Zhang PP, et al. MicroRNA-10a is involved in the metastatic process by regulating Eph tyrosine kinase receptor A4-mediated epithelial-mesenchymal transition and adhesion in hepatoma cells. Hepatology. 2013;57(2):667–77.
Takano H, Nakamura T, Tsuchikawa T, Kushibiki T, Hontani K, et al. Inhibition of Eph receptor A4 by 2,5-dimethylpyrrolyl benzoic acid suppresses human pancreatic cancer growing orthotopically in nude mice. Oncotarget. 2015;6(38):41063–76.
Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10(3):165–80.
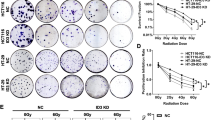
Bastos LG, de Marcondes PG, de-Freitas-Junior JC, Leve F, Mencalha AL, et al. Progeny from irradiated colorectal cancer cells acquire an EMT-like phenotype and activate Wnt/β-catenin pathway. J Cell Biochem. 2014;115(12):2175–87.
Flatmark K, Maelandsmo GM, Martinsen M, Rasmussen H, Fodstad Ø. Twelve colorectal cancer cell lines exhibit highly variable growth and metastatic capacities in an orthotopic model in nude mice. Eur J Cancer. 2004;40:1593–8.
Rowan AJ, Lamlum H, Ilyas M, Wheeler J, Straub J, Papadopoulou A, et al. APC mutations in sporadic colorectal tumors: a mutational “hotspot” and interdependence of the “two hits”. Proc Natl Acad Sci U S A. 2000;97:3352–7.
Petty A, Myshkin E, Qin H, Guo H, Miao H, et al. A small molecule agonist of EphA2 receptor tyrosine kinase inhibits tumor cell migration in vitro and prostate cancer metastasis in vivo. PLoS One. 2012;7(8):e42120.
Kandouz M. The Eph/Ephrin family in cancer metastasis: communication at the service of invasion. Cancer Metastasis. 2012;31(1–2):353–73.
Nievergall E, Lackmann M, Janes P. Eph-dependent cell-cell adhesion and segregation in development and cancer. Cell Mol Life Sci. 2011;11:1813–42.
Winning R, Wyman T, Walker G. EphA4 activity causes cell shape change and a loss of cell polarity in Xenopus laevis embryos. Differentiation. 2001;68(2–3):126–32.
Campbell P, Channing J. Oncogenic Ras and its role in tumor cell invasion and metastasis. Semin Cancer Biol. 2004;14(2):105–14.
Grille S, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, et al. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63(9):2172–8.
Anwar M, Kochhar R, Singh R, Bhatia A, Vaiphei K. Frequent activation of the β-catenin gene in sporadic colorectal carcinomas: a mutational & expression analysis. Mol Carcinog. 2015. doi:10.1002/mc.22414.
Malhotra P, Anwar M, Nanda N, Kochhar R, Wig JD. Alterations in K-ras, APC and p53-multiple genetic pathway in colorectal cancer among Indians. Tumour Biol. 2013;34(3):1901–11.
Liizumi M, Hosokawa M, Takehara A, Chung S, Nakamura T, et al. EphA4 receptor, overexpressed in pancreatic ductal adenocarcinoma, promotes cancer cell growth. Cancer Sci. 2006;97(11):1211–6.
Nojiri K, Iwakawa M, Ichikawa Y, Imadome K, Sakai M, et al. The proangiogenic factor ephrin-A1 is up-regulated in radioresistant murine tumor by irradiation. Exp Biol Med. 2009;234(1):112–22.
Liu C, Huang H, Wang C, Kong Y. Involvement of ephrin receptor A4 in pancreatic cancer cell motility and invasion. Oncol Lett. 2014;7(6):2165–9.
Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–29.
Ireton R, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5:149–57.
Orsulic S, Kemler R. Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J Cell Sci. 2000;113:1793–802.
Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–38.
Solanas G, Cortina C, Sevillano M, Batlle E. Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat Cell Biol. 2011;13(9):1100–7.
Batson J, MacCarthy-Morrogh L, Archer A, Tanton H, Nobes CD. EphA receptors regulate prostate cancer cell dissemination through Vav2–RhoA mediated cell–cell repulsion. Biol Open. 2004;3(6):453–62.
Oshima T, Akaike M, Yoshihara K, Shiozawa M, Yamamoto N, et al. Overexpression of EphA4 gene and reduced expression of EphB2 gene correlates with liver metastasis in colorectal cancer. Int J Oncol. 2008;33(3):573–7.
Shin J, Gu C, Kim J, Soochul P. Transient activation of the MAP kinase signaling pathway by the forward signaling of EphA4 in PC12 cells. BMP Rep. 2008;41(6):479–84.
Acknowledgments
This study was sponsored by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), Coordenação de Aperfeiçõamento de Pessoal de Nível Superior (CAPES), Ministério da Saúde – Brasil, Fundação Carlos Chagas Filho de Amparo á Pesquisa do Estado de Rio de Janeiro (FAPERJ), and Instituto Nacional de Ciência e Tecnologia em Câncer (573806/2008-0 and 170.026/2008). We are grateful to the Centro Nacional de Bioimagem (CENABIO) for the use of the super-resolution microscopy facility. This text was reviewed by American Journal Experts.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1 Supplemental
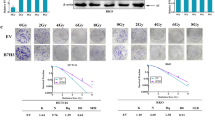
Total lysated from HT-29 F1 Cont and F1 5Gy were obtained and analyzed by immunoblotting for p-AKT and p-ERK. F1 5Gy cells show increased levels of these kinases comparing with F1 Cont cells. Bar graphs are plotted as a fold change of protein expression where non-treated cells (F1 Cont = 1). α-Tubulin was used as a loading control. Data are representative of two independent experiments (GIF 6 kb)
Rights and permissions
About this article
Cite this article
de Marcondes, P.G., Bastos, L.G., de-Freitas-Junior, J.C.M. et al. EphA4-mediated signaling regulates the aggressive phenotype of irradiation survivor colorectal cancer cells. Tumor Biol. 37, 12411–12422 (2016). https://doi.org/10.1007/s13277-016-5120-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5120-0