Abstract
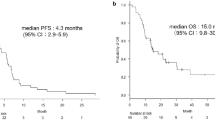
Several phase III clinical trials had authenticated that the addition of bevacizumab to paclitaxel plus carboplatin or gemcitabine plus cisplatin showed encouraging efficacy as first-line therapy for advanced NSCLC patients. However, the benefits of adding bevacizumab to other chemotherapy regimens in first- or second-line therapy have not been reported. To compare the clinical efficacy and safety of bevacizumab concomitant with chemotherapy regimens in patients with advanced NSCLC as first- or second-line therapy, we retrospectively reviewed the effects of adding bevacizumab to chemotherapy regimens in naive-chemotherapy and pre-chemotherapy patients with advanced non-squamous NSCLC. A total of 79 patients with advanced non-squamous NSCLC received at least two cycles of bevacizumab with chemotherapy between October 2010 and December 2013 were selected. Our primary end points were overall response rate (ORR) and disease control rate (DCR). The secondary objective was overall survival (OS) and safety. Seventy-nine patients were included in this study. Overall response rates at first evaluation (after 2 cycles) were 23.1 % (9/39) and 5.0 % (2/40) in first- and second-line therapy (P = 0.020), respectively. And disease control rates were 84.6 % (33/39) and 50 % (20/40), respectively (P = 0.001). The median OS were 27.2 months (95 % CI 13.3–41.1 months) and 29.6 months (95 % CI 6.7–52.5 months), respectively (P = 0.740). Grade 3–4 adverse events included leukopenia (2/39), and neutropenia (3/39) in first-line therapy versus neutropenia (1/40) and thrombocytopenia (2/40) in second-line treatment. In our experience, combination of bevacizumab and chemotherapy had encouraging anti-tumor efficacy as both first- and second-line therapy.

Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33:2197–204.
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAiL. J Clin Oncol. 2009;27:1227–34.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50.
Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–18.
Shepherd FA, Rodrigues PJ, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32.
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103.
Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62.
Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–91.
Liu KJ, Wu HY. A retrospective analysis of cisplatin, pemetrexed, and bevacizumab in previously treated non-small-cell lung cancer. Oncotarget 2015.
Adjei AA, Mandrekar SJ, Dy GK, Molina JR, Adjei AA, Gandara DR, et al. Phase II trial of pemetrexed plus bevacizumab for second-line therapy of patients with advanced non-small-cell lung cancer: NCCTG and SWOG study n0426. J Clin Oncol. 2010;28:614–9.
Heist RS, Fidias P, Huberman M, Ardman B, Sequist LV, Temel JS, et al. A phase II study of oxaliplatin, pemetrexed, and bevacizumab in previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:1153–8.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Ding L, Liu K, Jiang Z, Chen Q, Zhou N, Liang Y, et al. The efficacy and safety of pemetrexed plus bevacizumab in previously treated patients with advanced non-squamous non-small cell lung cancer (ns-NSCLC). Tumour Biol. 2015;36:2491–9.
Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007;25:4743–50.
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97.
Weiss JM, Stinchcombe TE. Second-line therapy for advanced NSCLC. Oncologist. 2013;18:947–53.
Barlesi F, Scherpereel A, Rittmeyer A, Pazzola A, Ferrer Tur N, Kim JH, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol. 2013;31:3004–11.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Microabstract
We performed the retrospective study to compare the clinical efficacy and safety of bevacizumab concomitant with chemotherapy regimens in patients with advanced NSCLC as first- or second-line therapy. In our experience, combination of bevacizumab and chemotherapy had encouraging anti-tumor efficacy as both first- and second-line therapy.
Rencui Quan and Jiaxing Huang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Quan, R., Huang, J., Chen, N. et al. A retrospective analysis of efficacy and safety of adding bevacizumab to chemotherapy as first- and second-line therapy in advanced non-small-cell lung cancer (NSCLC). Tumor Biol. 37, 11479–11484 (2016). https://doi.org/10.1007/s13277-016-5031-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5031-0