Abstract
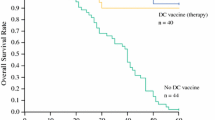
Dendritic cell (DC) vaccination targeting cancer stem cells is an effective way to suppress tumor progression and reduce the metastasis and recurrence. In the present study, we explored the suitability of side population (SP) cells as source of antigens for DC vaccination against hepatocellular carcinoma (HCC) in a mouse model. In this study, we identified the “stem-like” characteristics of SP cells in the MHCC97 and Hepa 1-6 HCC cell lines. We found that SP cells express high levels of tumor-associated antigens and MHC class I molecules. Although loading with cell lysates did not change the characteristics of DCs, SP cell lysate-pulsed DCs induced antigen-specific T cell responses, including T cell proliferation and increased IFN-γ production by stimulated CD8+ T cells. We investigated the cytotoxicity of T cells stimulated by SP cell lysate-pulsed DCs in nude mice co-injected with MHCC97 cells. To mimic the in vivo environment, we also confirmed the result in mouse HCC cell line Hepa 1-6 induced tumor-bearing C57/BL6 immune competent mice, and we demonstrated that vaccination with DCs loaded with Hepa 1-6 SP cell lysates could induce a T cell response in vivo and suppress the tumor growth. Our results may have applications for anti-HCC immunotherapy by targeting the cancer stem cells and may provide new insight for cancer vaccines.









Similar content being viewed by others
References
de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190–200.
Lau CK, Yang ZF, Fan ST. Role of stem cells in normal liver and cancer. Anticancer Agents Med Chem. 2011;11:522–8.
Palucka K, Banchereau J. Dendritic cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48.
Kandalaft LE, Chiang CL, Tanyi J, Motz G, Balint K, Mick R, et al. A Phase I vaccine trial using dendritic cells pulsed with autologous oxidized lysate for recurrent ovarian cancer. J Transl Med. 2013;11:149.
Kobayashi M, Sakabe T, Abe H, Tanii M, Takahashi H, Chiba A, et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg. 2013;17:1609–17.
Ernst B, Anderson KS. Immunotherapy for the treatment of breast cancer. Curr Oncol Rep. 2015;17:5.
Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–28.
Chiba T, Zheng YW, Kita K, Yokosuka O, Saisho H, Onodera M, et al. Enhanced self-renewal capability in hepatic stem/progenitor cells drives cancer initiation. Gastroenterology. 2007;133:937–50.
Qiu H, Fang X, Luo Q, Ouyang G. Cancer stem cells: a potential target for cancer therapy. Cell Mol Life Sci. 2015;72:3411–24.
Finocchiaro G, Pellegatta S. Immunotherapy with dendritic cells loaded with glioblastoma stem cells: From preclinical to clinical studies. Cancer Immunology, Immunotherapy. 2015;65:101–9.
Reyes D, Salazar L, Espinoza E, Pereda C, Castellón E, Valdevenito R, et al. Tumour cell lysate-loaded dendritic cell vaccine induces biochemical and memory immune response in castration-resistant prostate cancer patients. Brit J Cancer. 2013;109:1488–97.
Sun JC, Pan K, Chen MS, Wang QJ, Wang H, Ma HQ, et al. Dendritic cells-mediated CTLs targeting hepatocellular carcinoma stem cells. Cancer Biol Ther. 2010;10:368–75.
Chiba T, Kita K, Zheng Y, Yokosuka O, Saisho H, Iwama A, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–51.
Richard V, Nair MG, Santhosh Kumar TR, Pillai MR. Side population cells as prototype of chemoresistant, tumor-initiating cells. Biomed Res Int. 2013;2013:1–8.
Zhang N, Li R, Tao KS, Cao DY, Ti ZY, Ding R, et al. Characterization of a stem-like population in hepatocellular carcinoma MHCC97 cells. Oncol Rep. 2010;23:827–31.
Cao D, Yang J, Yue S, Tao K, Song Z, Wang D, et al. Comparative analysis of DC fused with allogeneic hepatocellular carcinoma cell line HepG2 and autologous tumor cells as potential cancer vaccines against hepatocellular carcinoma. Cell Immunol. 2009;259:13–20.
Shen H, Shao H, Chen X, Wu F, Wang H, Huang Z, et al. Identification of a novel HLA-A2-restricted mutated Survivin epitope and induction of specific anti-HCC CTLs that could effectively cross-recognize wild-type Survivin antigen. Cancer Immunol Immunother. 2013;62:393–403.
Ji J, Judkowski VA, Liu G, Wang H, Bunying A, Li Z, et al. Identification of novel human leukocyte antigen-A*0201-restricted, cytotoxic T lymphocyte epitopes on CD133 for cancer stem cell immunotherapy. Stem Cells Transl Med. 2014;3:356–64.
Trojan A, Witzens M, Schultze JL, Vonderheide RH, Harig S, Krackhardt AM, et al. Generation of cytotoxic T lymphocytes against native and altered peptides of human leukocyte antigen-A*0201 restricted epitopes from the human epithelial cell adhesion molecule. Cancer Res. 2001;61:4761–5.
Liu WH, Tao KS, You N, Liu ZC, Zhang HT, Dou KF. Differences in the properties and miRNA expression profiles between side populations from hepatic cancer cells and normal liver cells. PloS One. 2011;6:e23311.
Chen J, Guo XZ, Li HY, Wang D, Shao XD. Comparison of cytotoxic T lymphocyte responses against pancreatic cancer induced by dendritic cells transfected with total tumor RNA and fusion hybrided with tumor cell. Exp Biol Med. 2015;240:1310–8.
Islam F, Gopalan V, Smith RA, Lam AK. Translational potential of cancer stem cells: a review of the detection of cancer stem cells and their roles in cancer recurrence and cancer treatment. Exp Cell Res. 2015;335:135–47.
Pan K, Zhao JJ, Wang H, Li JJ, Liang XT, Sun JC, et al. Comparative analysis of cytotoxic T lymphocyte response induced by dendritic cells loaded with hepatocellular carcinoma-derived RNA or cell lysate. Int J Biol Sci. 2010;6:639–48.
Xu M, Yao Y, Hua W, Wu Z, Zhong P, Mao Y, et al. Mouse glioma immunotherapy mediated by A2B5+ GL261 cell lysate-pulsed dendritic cells. J Neurooncol. 2014;116:497–504.
Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells. 2009;27:1734–40.
Lu L, Tao H, Chang AE, Hu Y, Shu G, Chen Q, et al. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Oncoimmunology. 2015;4:e990767.
Pang YB, Zhong JH, Luo XL, Ou C, Guo Z, Xiang BD, Peng NF, Li LQ. Clinicopathological characteristics and liver stem cell marker expression in hepatocellular carcinoma involving bile duct tumor thrombi. Tumour Biol. 2015. doi:10.1007/s13277-015-4446-3.
Yin S, Li J, Hu C, Chen X, Yao M, Yan M, et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–50.
Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–4.
Lee MR, Ji SY, Mia-Jan K, Cho MY. Chemoresistance of CD133(+) colon cancer may be related with increased survivin expression. Biochem Biophys Res Commun. 2015;463:229–34.
Gires O, Klein CA, Baeuerle PA. On the abundance of EpCAM on cancer stem cells. Nat Rev Cancer. 2009;9:143. 143.
Pan Q, Pan K, Weng D, Zhao J, Zhang X, Wang D, et al. Annexin A3 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma. Mol Carcinogen. 2015;54:598–607.
Pan QZ, Pan K, Wang QJ, Weng DS, Zhao JJ, Zheng HX, et al. Annexin A3 as a potential target for immunotherapy of liver cancer stem-like cells. Stem Cells. 2015;33:354–66.
Wang B, Wang Q, Wang Z, Jiang J, Yu SC, Ping YF, et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014;74:5746–57.
Garrido G, Rabasa A, Garrido C, Lopez A, Chao L, Garcia-Lora AM, et al. Preclinical modeling of EGFR-specific antibody resistance: oncogenic and immune-associated escape mechanisms. Oncogene. 2014;33:3129–39.
Seliger B. Novel insights into the molecular mechanisms of HLA class I abnormalities. Cancer Immunol Immunother. 2012;61:249–54.
Acknowledgments
We thank Dr. Ben Ma and Wei Sun for their help in the experiment. The experiment was supported by National Natural Science Foundation (81201926).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiao Li, Zhuochao Zhang, Guoying Lin and Yuanxing Gao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, X., Zhang, Z., Lin, G. et al. Antigen-specific T cell response from dendritic cell vaccination using side population cell-associated antigens targets hepatocellular carcinoma. Tumor Biol. 37, 11267–11278 (2016). https://doi.org/10.1007/s13277-016-4935-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4935-z