Abstract
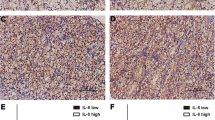
The aim of this study was to evaluate the potential prognostic significance of interleukin-33 (IL-33) in patients with clear-cell renal cell carcinoma (ccRCC) after surgical resection. In this retrospective research, we enrolled 203 patients with ccRCC undergoing nephrectomy between 2003 and 2004 in a single institution. We recorded clinicopathologic features, overall survival (OS), and recurrence-free survival (RFS) and assessed IL-33 expression by immunohistochemical staining. On such bases, the correlations between IL-33 expression and clinicopathologic features and prognosis were evaluated. A high expression of IL-33 was significantly associated with advanced TNM stage and Fuhrman grade (p = 0.017 and p < 0.001, respectively) in patients with ccRCC. Moreover, multivariate analysis identified IL-33 as an independent prognostic factor of OS for patients with ccRCC after surgery (hazard ratio = 2.050; 95 % CI 1.223–3.447; p = 0.006). The incorporation of IL-33 into the TNM stage and Fuhrman grade might help to refine individual risk stratification. The IL-33 expression may serve as an independent negative predictor of survival for patients with ccRCC after surgery.




Similar content being viewed by others
References
Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–21.
Oosterwijk E, Rathmell WK, Junker K, Brannon AR, Pouliot F, Finley DS, et al. Basic research in kidney cancer. Eur Urol. 2011;60:622–33.
Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205.
Lang H, Lindner W, de Fromont M, Molinie V, Letourneux H, Meyer N, et al. Multicenter determination of optimal interobserver agreement using the Fuhrman grading system for renal cell carcinoma—assessment of 241 patients with >15-year follow-up. Cancer. 2005;103:625–9.
Zisman A. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–66.
Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–400.
Sun M, Shariat SF, Cheng C, Ficarra V, Murai M, Oudard S, et al. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol. 2011;60:644–61.
Jovanovic IP, Pejnovic NN, Radosavljevic GD, Arsenijevic NN, Lukic ML. IL-33/ST2 axis in innate and acquired immunity to tumors. Oncoimmunol. 2012;1:229–31.
Zhao Q, Chen G. Role of IL-33 and its receptor in T cell-mediated autoimmune diseases. BioMed Res Int. 2014;2014:587376.
Jiang HR, Milovanovic M, Allan D, Niedbala W, Besnard AG, Fukada SY, et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-gamma production and inducing alternatively activated macrophages. Eur J Immunol. 2012;42:1804–14.
Schmitz J, Owyang A, Oldham E, Song YL, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90.
Miller AM. Role of IL-33 in inflammation and disease. J Inflamm. 2011;8:22.
Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3, e3331.
Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park H, et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114:3117–26.
Joshi AD, Oak SR, Hartigan AJ, Finn WG, Kunkel SL, Duffy KE, et al. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010;11:52.
Masamune A, Watanabe T, Kikuta K, Satoh K, Kanno A, Shimosegawa T. Nuclear expression of interleukin-33 in pancreatic stellate cells. Am J Physiol-Gastr L. 2010;299:G821–32.
Bergis D, Kassis V, Ranglack A, Koeberle V, Piiper A, Kronenberger B, et al. High serum levels of the interleukin 33 receptor soluble ST2 as a negative prognostic factor in hepatocellular carcinoma. J Hepatol. 2013;58:S257–7.
Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, et al. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer J Int Du Cancer. 2014;134:1669–82.
Hu L-A, Fu Y, Zhang D-N, Zhang J. Serum IL-33 as a diagnostic and prognostic marker in non-small cell lung cancer. Asian Pac J Cancer Prev. 2013;14:2563–6.
Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Pajares MJ, Agorreta J, Larrayoz M, Vesin A, Ezponda T, Zudaire I, et al. Expression of tumor-derived vascular endothelial growth factor and its receptors is associated with outcome in early squamous cell carcinoma of the lung. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:1129–36.
Mager LF, Riether C, Schurch CM, Banz Y, Wasmer MH, Stuber R, et al. IL-33 signaling contributes to the pathogenesis of myeloproliferative neoplasms. J Clin Invest. 2015;125:2579–91.
Yu XX, Hu Z, Shen X, Dong LY, Zhou WZ, Hu WH. IL-33 promotes gastric cancer cell invasion and migration via ST2-ERK1/2 pathway. Dig Dis Sci. 2015;60:1265–72.
Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–40.
Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6.
Jovanovic I, Radosavljevic G, Mitrovic M, Juranic VL, McKenzie AN, Arsenijevic N, et al. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur J Immunol. 2011;41:1902–12.
Acknowledgments
This study was funded by grants from the National Basic Research Program of China (2012CB822104), National Key Projects for Infectious Diseases of China (2012ZX10002012-007, 2016ZX10002018-008), National Natural Science Foundation of China (31100629, 31270863, 81372755, 31470794, 81401988, 81402082, 81402085, 81471621, 81472227, 81472376, 31570803, 81501999, and 81572352), and Program for New Century Excellent Talents in University (NCET-13-0146). All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Zewei Wang, Le Xu and Yuan Chang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Kaplan-Meier analysis of OS in patients with TNM stage III (a) and stage IV (b) based on the expression of IL-33. Kaplan-Meier analysis of RFS in patients with T3-4N0M0 (c). P value was calculated by log-rank test. (GIF 16 kb)
Rights and permissions
About this article
Cite this article
Wang, Z., Xu, L., Chang, Y. et al. IL-33 is associated with unfavorable postoperative survival of patients with clear-cell renal cell carcinoma. Tumor Biol. 37, 11127–11134 (2016). https://doi.org/10.1007/s13277-016-4879-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4879-3