Abstract
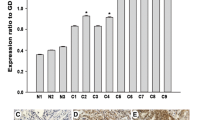
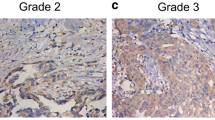
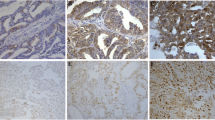
This study is designated to investigate the roles of cyclin Y (CCNY) and Wnt signaling pathway in regulating ovarian cancer (OC) cell proliferation, migration, and invasion. Quantitative real-time PCR (qRT-PCR), Western blot, MTT assay, cell scratch, and transwell test were used in our study, and transplanted tumor model was constructed on nude mice. C-Myc, cyclin D1, PFTK1, ki67, OGT, and β-catenin protein expressions in tumor tissues were detected. CCNY was significantly upregulated in OC cell lines and tissues (both P < 0.05); significant association was observed between CCNY expression and clinicopathological stage, lymph node metastasis (LNM) (P < 0.05); and the CCNY expression in stages III to IV was higher than that in stages I to II, and patients with LNM had higher CCNY expression when compared with those in patients without LNM (P < 0.05); expressions of c-Myc, cyclin D, PFTK1, ki67, and OGT were upregulated in OC tissues compared with ovarian benign tissues, suggesting that these expressions were significantly different between the two groups (P < 0.05); CCNY significantly exacerbated proliferation, migration, and invasion of A2780 cells; c-Myc and cyclin D1 protein expressions increased as the expression of CCNY increased (P < 0.001); β-catenin expressions in A2780 cells with over-expression of CCNY were significantly increased in the nucleus, but significantly decreased in the cytoplasm (both P < 0.05); high expressions of CCNY exacerbated the proliferation of A2780 cells in nude mice and significantly increased c-Myc, cyclin D1, PFTK1, ki67, and OGT protein expressions in tumor tissues which were transplanted into nude mice (P < 0.01). CCNY might exacerbate the proliferation, migration, and invasion of OC cells via activating the Wnt signaling pathway. Thus, this study provides a theoretical foundation for the development of therapeutic drugs that are able to cure OC by targeting CCNY.










Similar content being viewed by others
References
Gao L, Ye X, Ma RQ, Cheng HY, Han HJ, Cui H, et al. Low programmed cell death 5 expression is a prognostic factor in ovarian cancer. Chin Med J (Engl). 2015;128(8):1084–90.
Liu J, Matulonis UA. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin Cancer Res. 2014;20(20):5150–6.
Gao J, Yang X, Zhang Y. Systematic lymphadenectomy in the treatment of epithelial ovarian cancer: a meta-analysis of multiple epidemiology studies. Jpn J Clin Oncol. 2015;45(1):49–60.
Quitadamo A, Tian L, Hall B, Shi X. An integrated network of microRNA and gene expression in ovarian cancer. BMC Bioinformatics. 2015;16 Suppl 5:S5.
Liu H, Yan ZQ, Li B, Yin SY, Sun Q, Kou JJ, et al. Reduced expression of SOX7 in ovarian cancer: a novel tumor suppressor through the Wnt/beta-catenin signaling pathway. J Ovarian Res. 2014;7:87.
Mostowska A, Pawlik P, Sajdak S, Markowska J, Pawalowska M, Lianeri M, et al. An analysis of polymorphisms within the Wnt signaling pathway in relation to ovarian cancer risk in a Polish population. Mol Diagn Ther. 2014;18(1):85–91.
Jiang M, Gao Y, Yang T, Zhu X, Chen J. Cyclin Y, a novel membrane-associated cyclin, interacts with PFTK1. FEBS Lett. 2009;583(13):2171–8.
Cho E, Kim DH, Hur YN, Whitcomb DJ, Regan P, Hong JH, et al. Cyclin Y inhibits plasticity-induced AMPA receptor exocytosis and LTP. Sci Rep. 2015;5(12):624.
An W, Zhang Z, Zeng L, Yang Y, Zhu X, Wu J. Cyclin Y is involved in the regulation of adipogenesis and lipid production. PLoS One. 2015;10(7):e0132721.
D’Addabbo A, Palmieri O, Maglietta R, Latiano A, Mukherjee S, Annese V, et al. Discovering genetic variants in Crohn’s disease by exploring genomic regions enriched of weak association signals. Dig Liver Dis. 2011;43(8):623–31.
Yue W, Zhao X, Zhang L, Xu S, Liu Z, Ma L, et al. Cell cycle protein cyclin Y is associated with human non-small-cell lung cancer proliferation and tumorigenesis. Clin Lung Cancer. 2011;12(1):43–50.
Ma L, Yue W, Teng Y, Zhang L, Gu M, Wang Y. Serum anti-CCNY autoantibody is an independent prognosis indicator for postoperative patients with early-stage nonsmall-cell lung carcinoma. Dis Markers. 2013;35(5):317–25.
Sun T, Co NN, Wong N. PFTK1 interacts with cyclin Y to activate non-canonical Wnt signaling in hepatocellular carcinoma. Biochem Biophys Res Commun. 2014;449(1):163–8.
Ford CE, Jary E, Ma SS, Nixdorf S, Heinzelmann-Schwarz VA, Ward RL. The Wnt gatekeeper SFRP4 modulates EMT, cell migration and downstream Wnt signalling in serous ovarian cancer cells. PLoS One. 2013;8(1):e54362.
M PN. World Medical Association publishes the revised declaration of Helsinki. Natl Med J India. 2014;27(1):56.
Zhao X, Jiang M, Yue W. The function and molecular mechnism of cyclin Y protein. Zhongguo Fei Ai Za Zhi. 2013;16(11):605–8.
Tai J, Li AD, Rao YS, Huang YB, Huang ZG, Yu ZK, et al. Influence on cell proliferation by small interfering RNA of Cyclin Y expression in laryngeal cancer cells. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;48(9):761–4.
Xu Y, Wang Z, Wang J, Li J, Wang H, Yue W. Lentivirus-mediated knockdown of cyclin Y (CCNY) inhibits glioma cell proliferation. Oncol Res. 2010;18(8):359–64.
Fan S, Zhao C, Zhang L, Dai S, Ren J, Zhang X, et al. Knockdown of PFTK1 inhibits the migration of glioma cells. J Mol Neurosci. 2015;57(2):257–64.
Miyagaki H, Yamasaki M, Miyata H, Takahashi T, Kurokawa Y, Nakajima K, et al. Overexpression of PFTK1 predicts resistance to chemotherapy in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2012;106(5):947–54.
Battista MJ, Mantai N, Sicking I, Cotarelo C, Weyer V, Lebrecht A, et al. Ki-67 as an independent prognostic factor in an unselected cohort of patients with ovarian cancer: results of an explorative, retrospective study. Oncol Rep. 2014;31(5):2213–9.
Liu P, Sun YL, Du J, Hou XS, Meng H. CD105/Ki67 coexpression correlates with tumor progression and poor prognosis in epithelial ovarian cancer. Int J Gynecol Cancer. 2012;22(4):586–92.
Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V, et al. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res. 2013;73(16):5277–87.
Krzeslak A, Forma E, Bernaciak M, Romanowicz H, Brys M. Gene expression of O-GlcNAc cycling enzymes in human breast cancers. Clin Exp Med. 2012;12(1):61–5.
Liu D, Guest S, Finley Jr RL. Why Cyclin Y? A highly conserved cyclin with essential functions. Fly (Austin). 2010;4(4):278–82.
Davidson G, Niehrs C. Emerging links between CDK cell cycle regulators and Wnt signaling. Trends Cell Biol. 2010;20(8):453–60.
Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, et al. Cell cycle control of Wnt receptor activation. Dev Cell. 2009;17(6):788–99.
Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20(2):119–25.
van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–14.
Davidson G. The cell cycle and Wnt. Cell Cycle. 2010;9(9):1667–8.
Koehler A, Schlupf J, Schneider M, Kraft B, Winter C, Kashef J. Loss of Xenopus cadherin-11 leads to increased Wnt/beta-catenin signaling and up-regulation of target genes c-myc and cyclin D1 in neural crest. Dev Biol. 2013;383(1):132–45.
Shan BE, Wang MX, Li RQ. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/beta-catenin signaling pathway. Cancer Invest. 2009;27(6):604–12.
Hendaoui I, Tucker RP, Zingg D, Bichet S, Schittny J, Chiquet-Ehrismann R. Tenascin-C is required for normal Wnt/beta-catenin signaling in the whisker follicle stem cell niche. Matrix Biol. 2014;40:46–53.
Li X, Chen C, Wang F, Huang W, Liang Z, Xiao Y, et al. KCTD1 suppresses canonical Wnt signaling pathway by enhancing beta-catenin degradation. PLoS One. 2014;9(4):e94343.
Acknowledgments
We would like to express our sincere appreciation to the reviewers for their constructive comments on this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental protocol was approved by the Ethics Committee of Animal Experiment Center of Peking Union Medical College Hospital and complied with the standards with respect to animal experiment published by the US National Institutes of Health in 1996.
Conflicts of interest
None
Ethics approval and informed consent
This study was approved by the ethics committee of Peking Union Medical College Hospital, and all patients signed the informed consent. The implementation of this study complied with the standards set by the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Liu, H., Shi, H., Fan, Q. et al. Cyclin Y regulates the proliferation, migration, and invasion of ovarian cancer cells via Wnt signaling pathway. Tumor Biol. 37, 10161–10175 (2016). https://doi.org/10.1007/s13277-016-4818-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4818-3