Abstract
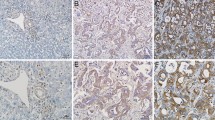
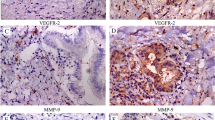
LIM and SH3 protein 1 (LASP-1) is demonstrated to play a key role in occurrence and development of tumors. However, the expression and function of LASP-1 in cholangiocarcinoma (CCA) remain largely unexplored. This study aimed to investigate the effect of regulated LASP-1 expression on migration, invasion, proliferation, and apoptosis of CCA cells and on tumorigenesis in vivo, and to examine clinico-oncological correlates of LASP-1 expression. Expression of LASP-1 by immunohistochemistry was evaluated in CCA tissue samples. HCCC-9810 and RBE cells were transfected with the LASP-1 small interfering RNA (siRNA), and the effect of knocking down LASP-1 gene expression on cell migration, invasion, proliferation, and apoptosis were examined by wound healing, transwell assays, CCK-8 assays, colony formation, and flow cytometry assays, respectively. Xenograft tumor model was used to validate the effect of downregulated LASP-1 in vivo. Our results demonstrated that LASP-1 was over-expressed in CCA tissues, positively correlating with larger tumors, poor histological differentiation, lymph node metastasis, advanced TNM stage, and poor prognosis in CCA patients (P < 0.05). Downregulation of LASP-1 in HCCC-9810 and RBE cell lines significantly increased cell apoptosis and suppressed cell migration, invasion, and proliferation in vitro and tumorigenesis in vivo. Our results indicate that LASP-1 may essentially involve in the metastasis and growth of CCA and clinical significance of LASP-1 may reside in function as a biomarker to predict prognosis and as a promising therapeutic strategy for CCA patients by the inhibition of LASP-1 expression.





Similar content being viewed by others
References
Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:221–32.
Schweitzer N, Vogel A. Systemic therapy of cholangiocarcinoma: from chemotherapy to targeted therapies. Best Pract Res Clin Gastroenterol. 2015;29:345–53.
Kongpetch S, Jusakul A, Ong CK, Lim WK, Rozen SG, Tan P, et al. Pathogenesis of cholangiocarcinoma: from genetics to signalling pathways. Best Pract Res Clin Gastroenterol. 2015;29:233–44.
Tomasetto C, Regnier C, Moog-Lutz C, Mattei MG, Chenard MP, Lidereau R, et al. Identification of four novel human genes amplified and overexpressed in breast carcinoma and localized to the q11-q21.3 region of chromosome 17. Genomics. 1995;28:367–76.
Pappas CT, Bliss KT, Zieseniss A, Gregorio CC. The nebulin family: an actin support group. Trends Cell Biol. 2011;21:29–37.
Grunewald TG, Butt E. The lim and sh3 domain protein family: structural proteins or signal transducers or both? Mol Cancer. 2008;7:31.
Frietsch JJ, Grunewald TG, Jasper S, Kammerer U, Herterich S, Kapp M, et al. Nuclear localisation of lasp-1 correlates with poor long-term survival in female breast cancer. Br J Cancer. 2010;102:1645–53.
Takeshita N, Mori M, Kano M, Hoshino I, Akutsu Y, Hanari N, et al. Mir-203 inhibits the migration and invasion of esophageal squamous cell carcinoma by regulating lasp1. Int J Oncol. 2012;41:1653–61.
Zheng J, Yu S, Qiao Y, Zhang H, Liang S, Wang H, et al. Lasp-1 promotes tumor proliferation and metastasis and is an independent unfavorable prognostic factor in gastric cancer. J Cancer Res Clin Oncol. 2014;140:1891–9.
Salvi A, Bongarzone I, Ferrari L, Abeni E, Arici B, De Bortoli M, et al. Molecular characterization of lasp-1 expression reveals vimentin as its new partner in human hepatocellular carcinoma cells. Int J Oncol. 2015;46:1901–12.
Zhao T, Ren H, Li J, Chen J, Zhang H, Xin W, et al. Lasp1 is a hif1alpha target gene critical for metastasis of pancreatic cancer. Cancer Res. 2015;75:111–9.
Orth MF, Cazes A, Butt E, Grunewald TG. An update on the lim and sh3 domain protein 1 (lasp1): a versatile structural, signaling, and biomarker protein. Oncotarget. 2015;6:26–42.
Opferman JT. Attacking cancer’s Achilles heel: antagonism of anti-apoptotic bcl-2 family members. FEBS J. 2015.
Zhu AX. Future directions in the treatment of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:355–61.
Zhao L, Wang H, Liu C, Liu Y, Wang X, Wang S, et al. Promotion of colorectal cancer growth and metastasis by the lim and sh3 domain protein 1. Gut. 2010;59:1226–35.
Ardelt P, Grunemay N, Strehl A, Jilg C, Miernik A, Kneitz B, et al. Lasp-1, a novel urinary marker for detection of bladder cancer. Urol Oncol. 2013;31:1591–8.
Wang H, Li W, Jin X, Cui S, Zhao L. Lim and sh3 protein 1, a promoter of cell proliferation and migration, is a novel independent prognostic indicator in hepatocellular carcinoma. Eur J Cancer (Oxford, England : 1990). 2013;49:974–83.
Grunewald TG, Kammerer U, Schulze E, Schindler D, Honig A, Zimmer M, et al. Silencing of lasp-1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp Cell Res. 2006;312:974–82.
Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–40.
Chou YS, Yang MH. Epithelial-mesenchymal transition-related factors in solid tumor and hematological malignancy. J Chin Med Assoc. 2015;78:438–45.
Wang H, Shi J, Luo Y, Liao Q, Niu Y, Zhang F, et al. Lim and sh3 protein 1 induces tgfbeta-mediated epithelial-mesenchymal transition in human colorectal cancer by regulating s100a4 expression. Clin Cancer Res. 2014;20:5835–47.
Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16:2129–44.
Delbridge AR, Strasser A. The bcl-2 protein family, bh3-mimetics and cancer therapy. Cell Death Differ. 2015;22:1071–80.
Li B, Zhuang L, Trueb B. Zyxin interacts with the sh3 domains of the cytoskeletal proteins lim-nebulette and lasp-1. J Biol Chem. 2004;279:20401–10.
Grunewald TG, Kammerer U, Winkler C, Schindler D, Sickmann A, Honig A, et al. Overexpression of lasp-1 mediates migration and proliferation of human ovarian cancer cells and influences zyxin localisation. Br J Cancer. 2007;96:296–305.
Choi YH, McNally BT, Igarashi P. Zyxin regulates migration of renal epithelial cells through activation of hepatocyte nuclear factor-1beta. Am J Physiol Renal Physiol. 2013;305:F100–10.
Acknowledgments
This study was supported by the Introductory Funding project from Shanghai Science and Technology Bureau (124119a0600) to WG and Science and Technology funding project of Shanghai Jiao Tong University, School of Medicine (13xj22003) to BFC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was performed with approval of the Ethics Committee of Xinhua Hospital and the ethical guidelines of the Declaration of Helsinki. In addition, written informed consent was obtained from all participants.
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University (Shanghai, China).
Conflicts of interest
None
Additional information
Hongchen Zhang, Zhizhen Li and Bingfeng Chu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, H., Li, Z., Chu, B. et al. Upregulated LASP-1 correlates with a malignant phenotype and its potential therapeutic role in human cholangiocarcinoma. Tumor Biol. 37, 8305–8315 (2016). https://doi.org/10.1007/s13277-015-4704-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4704-4